よむ、つかう、まなぶ。
資料1-3 ニフェジピン 調査結果報告書及び添付文書 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
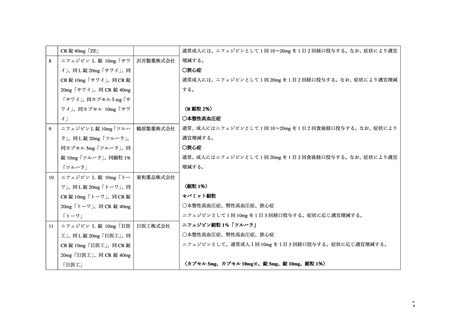
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
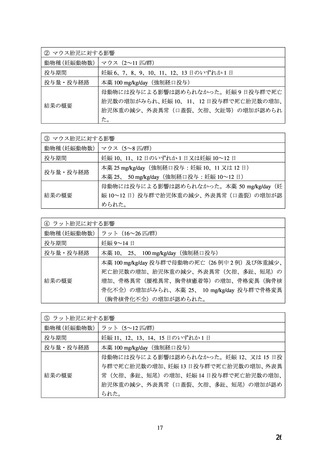
Rat:
Oral: 1022 (950-1087)*;
i.v.: 15.5 (13.7-17.5)*.
Rabbit
Oral: 250-500;
i.v.: 2-3.
Cat:
Oral: ~ 100;
i.v.: 0.5-8.
Dog:
Oral: > 250;
i.v.: 2-3.
* 95% confidence interval.
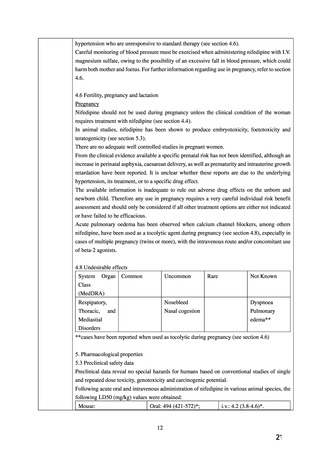
In subacute and subchronic toxicity studies in rats and dogs, nifedipine was tolerated without
damage at doses of up to 50 mg/kg (rats) and 100 mg/kg (dogs) p.o. over periods of thirteen and
four weeks, respectively. Following intravenous administration, dogs tolerated up to 0.1 mg/kg
nifedipine for six days without damage. Rats tolerated daily intravenous administration of 2.5
mg/kg nifedipine over a period of three weeks without damage.
In chronic toxicity studies in dogs with treatment lasting up to one year, nifedipine was tolerated
without damage at doses up to and including 100 mg/kg p.o. In rats, toxic effects occurred at
concentrations above 100 ppm in the feed (approximately 5-7 mg/kg bodyweight).
In a carcinogenicity study in rats (two years), there was no evidence of a carcinogenic effect of
nifedipine.
Nifedipine has been shown to produce teratogenic findings in rats, mice and rabbits, including
digital anomalies, malformation of the extremities, cleft palates, cleft sternum and malformation
of the ribs.
Digital anomalies and malformation of the extremities are possibly a result of compromised
uterine blood flow, but have also been observed in animals treated with nifedipine solely after
the end of the organogenesis period.
Nifedipine administration was associated with a variety of embryotoxic, placentotoxic and
foetotoxic effects, including stunted foetuses (rats, mice, rabbits), small placentas and
underdeveloped chorionic villi (monkeys), embryonic and foetal deaths (rats, mice, rabbits) and
prolonged pregnancy/decreased neonatal survival (rats; not evaluated in other species). The risk
to humans cannot be ruled out if a sufficiently high systemic exposure is achieved, however, all
of the doses associated with the teratogenic, embryotoxic or foetotoxic effects in animals were
maternally toxic and were several times the recommended maximum dose for humans.
In in vitro and in vivo tests, nifedipine has not been associated with mutagenic properties.
備考
2006 年時点での英国添付文書記載は、妊娠 20 週以降の妊婦に対してはリスク・ベネ
フィットを慎重に評価して他の治療方法が適切でない、あるいは有効でない場合に限
定して使用することとされ、妊娠 20 週未満は妊婦禁忌とされていた。2012 年から
EU 規制当局による統一評価手続きがなされ、2014 年 1 月 22 日に妊娠 20 週未満の妊
婦についての記載を「Contraindications」から削除し、
「Special warnings and precautions
for use」に注意としての記載に変更された。
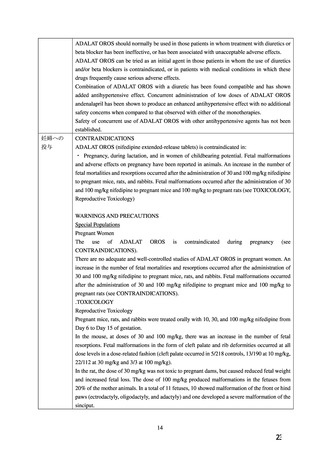
経口剤(加国)
(3) 製品名 ADALAT OROS (nifedipine extended-release tablets))/ Bayer Inc.
効能・効果
INDICATIONS AND CLINICAL USE
ADALAT OROS (nifedipine extended-release tablets) is indicated for::
Chronic Stable Angina
Hypertension
ADALAT OROS is indicated in the management of mild to moderate essential hypertension.
13
22
Oral: 1022 (950-1087)*;
i.v.: 15.5 (13.7-17.5)*.
Rabbit
Oral: 250-500;
i.v.: 2-3.
Cat:
Oral: ~ 100;
i.v.: 0.5-8.
Dog:
Oral: > 250;
i.v.: 2-3.
* 95% confidence interval.
In subacute and subchronic toxicity studies in rats and dogs, nifedipine was tolerated without
damage at doses of up to 50 mg/kg (rats) and 100 mg/kg (dogs) p.o. over periods of thirteen and
four weeks, respectively. Following intravenous administration, dogs tolerated up to 0.1 mg/kg
nifedipine for six days without damage. Rats tolerated daily intravenous administration of 2.5
mg/kg nifedipine over a period of three weeks without damage.
In chronic toxicity studies in dogs with treatment lasting up to one year, nifedipine was tolerated
without damage at doses up to and including 100 mg/kg p.o. In rats, toxic effects occurred at
concentrations above 100 ppm in the feed (approximately 5-7 mg/kg bodyweight).
In a carcinogenicity study in rats (two years), there was no evidence of a carcinogenic effect of
nifedipine.
Nifedipine has been shown to produce teratogenic findings in rats, mice and rabbits, including
digital anomalies, malformation of the extremities, cleft palates, cleft sternum and malformation
of the ribs.
Digital anomalies and malformation of the extremities are possibly a result of compromised
uterine blood flow, but have also been observed in animals treated with nifedipine solely after
the end of the organogenesis period.
Nifedipine administration was associated with a variety of embryotoxic, placentotoxic and
foetotoxic effects, including stunted foetuses (rats, mice, rabbits), small placentas and
underdeveloped chorionic villi (monkeys), embryonic and foetal deaths (rats, mice, rabbits) and
prolonged pregnancy/decreased neonatal survival (rats; not evaluated in other species). The risk
to humans cannot be ruled out if a sufficiently high systemic exposure is achieved, however, all
of the doses associated with the teratogenic, embryotoxic or foetotoxic effects in animals were
maternally toxic and were several times the recommended maximum dose for humans.
In in vitro and in vivo tests, nifedipine has not been associated with mutagenic properties.
備考
2006 年時点での英国添付文書記載は、妊娠 20 週以降の妊婦に対してはリスク・ベネ
フィットを慎重に評価して他の治療方法が適切でない、あるいは有効でない場合に限
定して使用することとされ、妊娠 20 週未満は妊婦禁忌とされていた。2012 年から
EU 規制当局による統一評価手続きがなされ、2014 年 1 月 22 日に妊娠 20 週未満の妊
婦についての記載を「Contraindications」から削除し、
「Special warnings and precautions
for use」に注意としての記載に変更された。
経口剤(加国)
(3) 製品名 ADALAT OROS (nifedipine extended-release tablets))/ Bayer Inc.
効能・効果
INDICATIONS AND CLINICAL USE
ADALAT OROS (nifedipine extended-release tablets) is indicated for::
Chronic Stable Angina
Hypertension
ADALAT OROS is indicated in the management of mild to moderate essential hypertension.
13
22