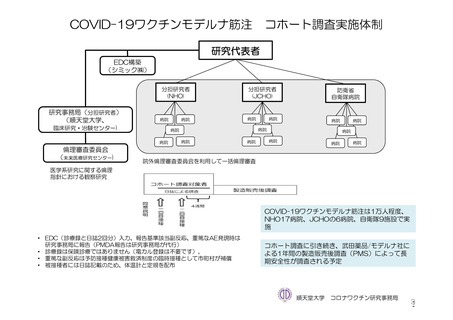
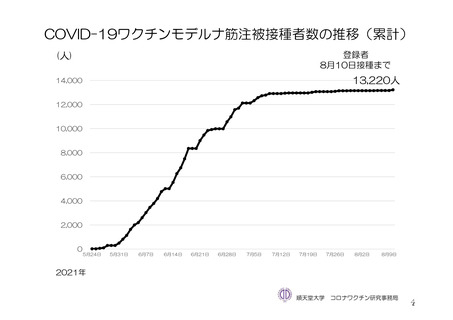
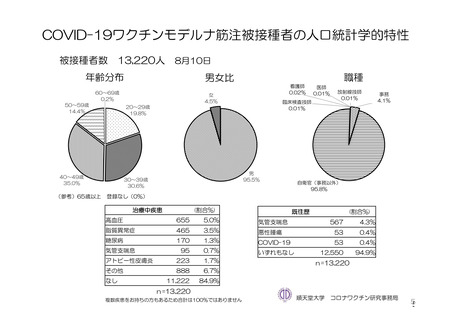
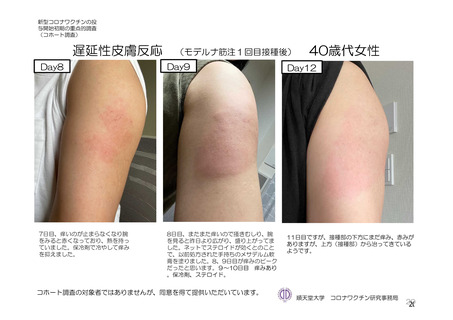
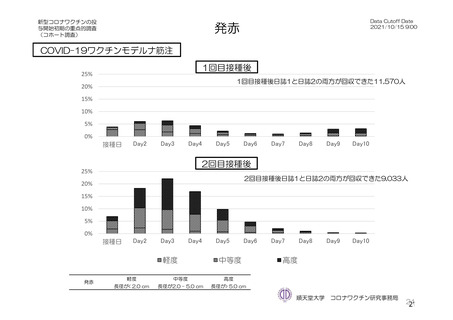
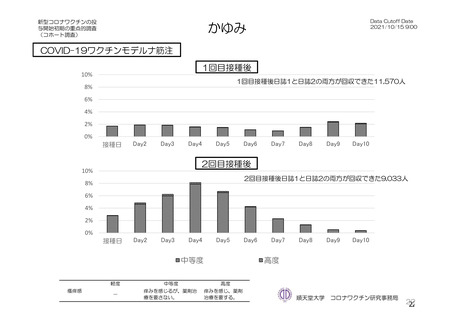
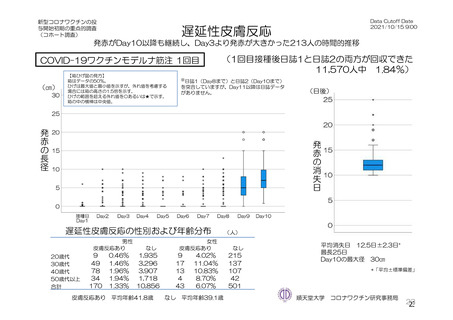
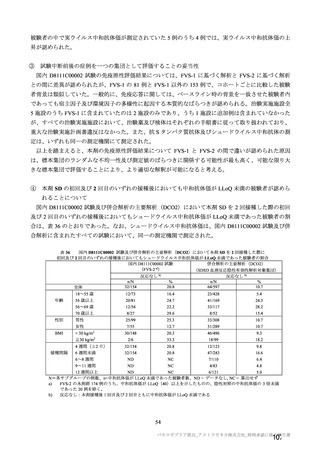
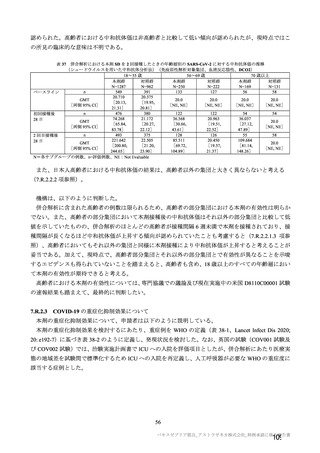
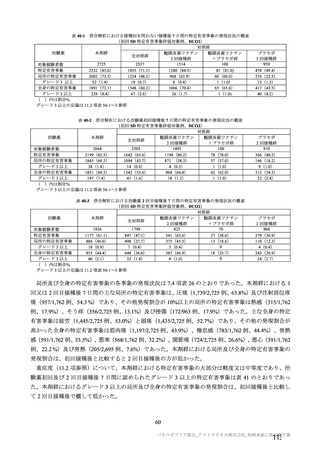
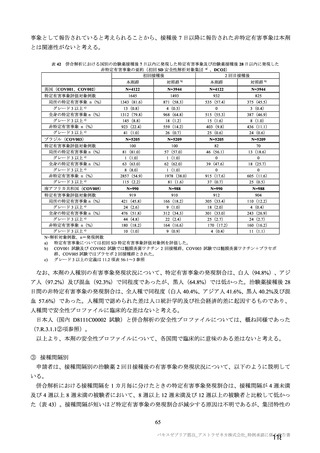
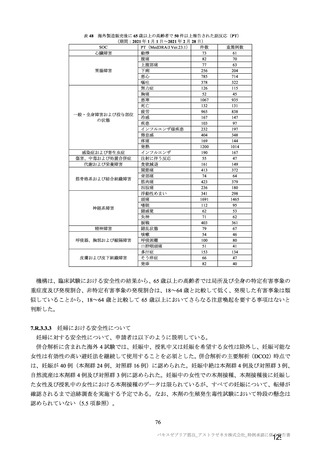
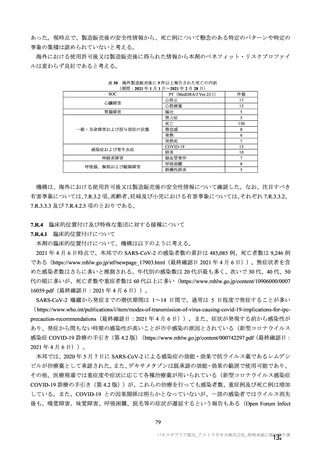
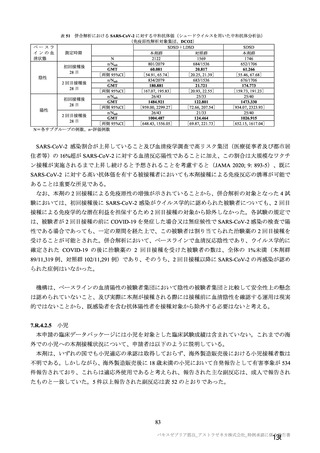
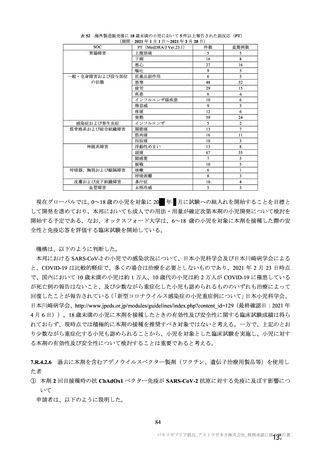
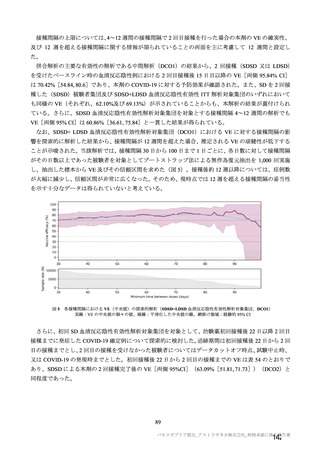
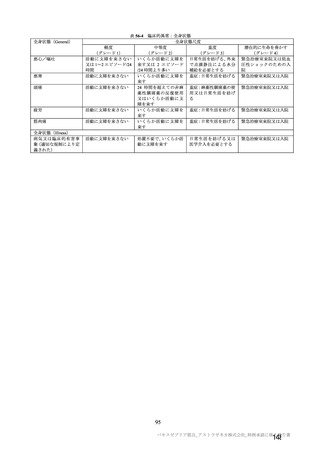
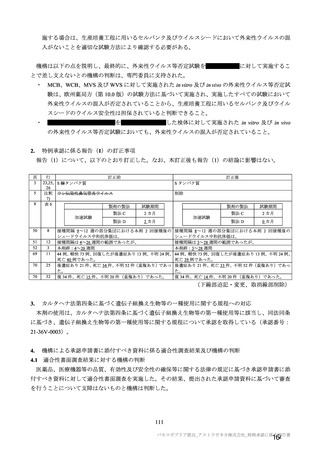
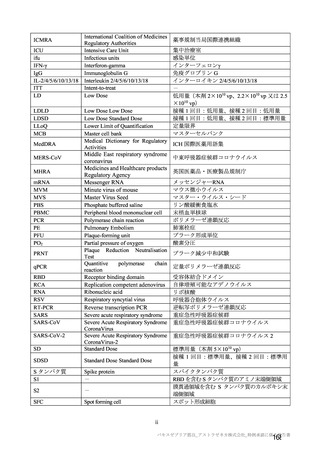
参考資料1-7 浜口班の議論における参考資料(令和3年10月25日開催)(令和3年度第6回安全技術調査会参考資料1-2) (195 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_27504.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会安全技術調査会(令和4年度第2回 8/23)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
2021 DOI:10.1056/NEJMe2106315
12. https://www.nature.com/articles/d41586-021-00932-0(2021/05/26 アクセス)
13. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1S [recombinant]) –COVID-19 Vaccine AstraZeneca (Other viral vaccines). EPITT no:19683
https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-reportembolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf(2021/05/26
アクセス)
14. Vaxzevria (COVID-19 Vaccine (ChAdOx1-S [recombinant])). An overview of Vaxzevria and why it is
authorised in the EU. https://www.ema.europa.eu/en/documents/overview/vaxzevria-previouslycovid-19-vaccine-astrazeneca-epar-medicine-overview_en.pdf(2021/05/26 アクセス)
15. Greinacher A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015; 373: 25261. doi: 10.1056/NEJMcp1411910.
16. Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparininduced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408
patients. Thromb Haemost. 2005; 94: 132-135. doi: 10.1160/TH04-12-0825.
17. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb
Haemost. 2017; 15: 2099-2114. doi: 10.1111/jth.13813.
18. Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag FG, Weißenborn K,
Höglinger GU, Maasoumy B, Wedemeyer H, Ganser A. Prothrombotic immune thrombocytopenia after
COVID-19 vaccine. Blood. 2021 Apr 28. doi: 10.1182/blood.2021011958.
19. Platton S, Bartlett A, MacCallum P, Makris M, McDonald V, Singh D, Scully M, Pavord S. Evaluation of
laboratory assays for anti-Platelet Factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb
Haemost. 2021 May 10. doi: 10.1111/jth.15362.
20. Thiele T, Ulm L, Holtfreter S, Schönborn L, Kuhn SO, Scheer C, Warkentin TE, Bröker B, Becker K, Aurich
K, Selleng K, Hübner NO, Greinacher A. Frequency of positive anti-PF4/polyanion antibody tests after
COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021 May 14; blood.2021012217.
doi: 10.1182/blood.2021012217.
21. Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Bryant BJ, Alperin JB,
Deloughery TG, Mulvey KP, Dhakal B, Wen R, Wang D, Aster RH. IVIg for Treatment of Severe Refractory
Heparin-Induced Thrombocytopenia. Chest. 2017; 152: 478-485. doi: 10.1016/j.chest.2017.03.050.
22. Warkentin TE, Climans TH, Morin P-A: Intravenous Immune Globulin to Prevent Heparin-Induced
Thrombocytopenia. N Engl J Med. 2018; 378: 1845-1848. doi: 10.1056/NEJMc1801799.
23. Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparininduced
thrombocytopenia:
a
review.
Expert
Rev
Hematol.
2019;
12:
685-698.
doi:
10.1080/17474086.2019.1636645.
19
195