よむ、つかう、まなぶ。
参考資料1-2 TERMS®サリドマイド製剤等安全管理手順[6.2MB] (2 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_40908.html |
| 出典情報 | 薬事審議会 医薬品等安全対策部会安全対策調査会(令和6年度第3回 6/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
目次
1.背景 ··························································································
1
2.目的 ··························································································
1
3.用語の定義 ·················································································
2
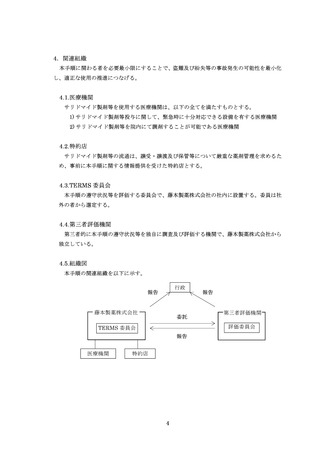
4.関連組織 ····················································································
4
4.1.医療機関 ·············································································
4
4.2.特約店·················································································
4
4.3.TERMS 委員会 ·····································································
4
4.4.第三者評価機関 ····································································
4
4.5.組織図·················································································
4
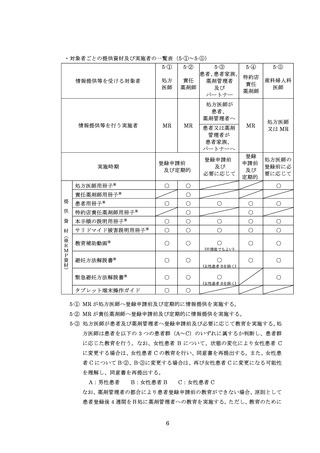
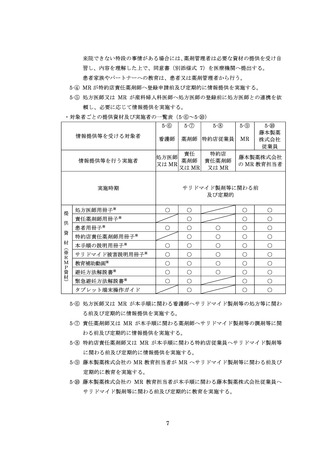
5.情報提供及び教育 ········································································
5
5.1.対象者·················································································
5
5.2.実施方法 ·············································································
5
6.登録 ··························································································
9
6.1.登録対象者 ··········································································
9
6.2.登録要件 ·············································································
9
6.3.登録手順·············································································
11
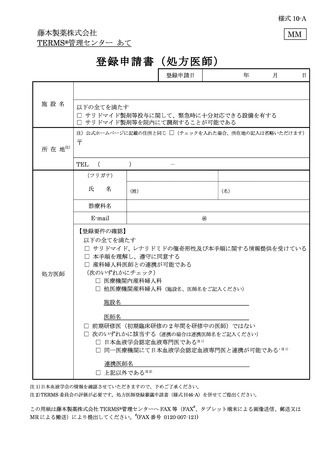
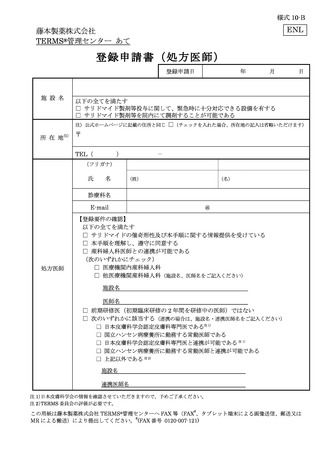
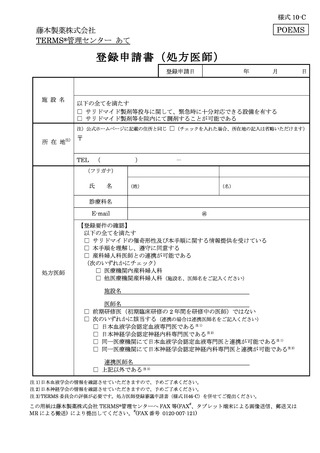
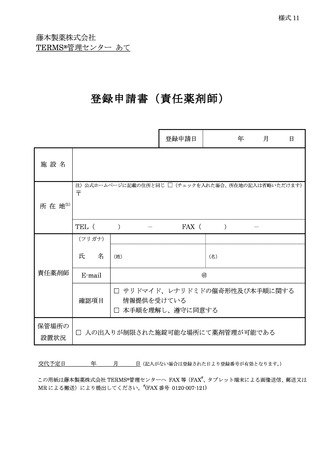
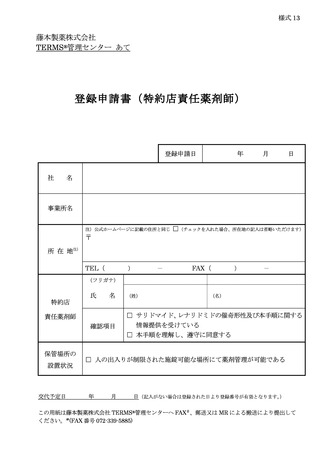
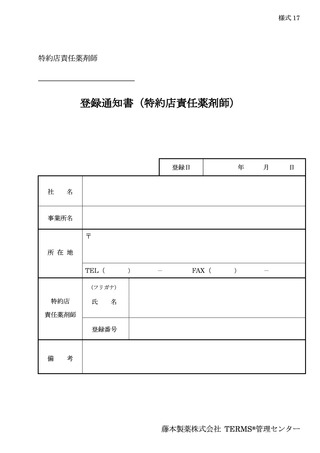
6.3.1.登録申請 ········································································ 11
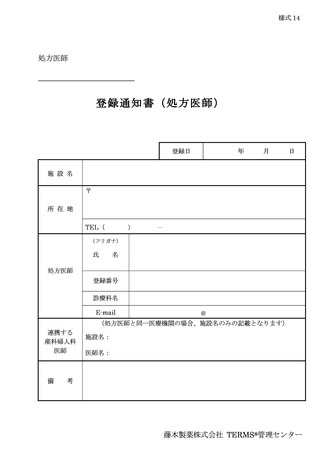
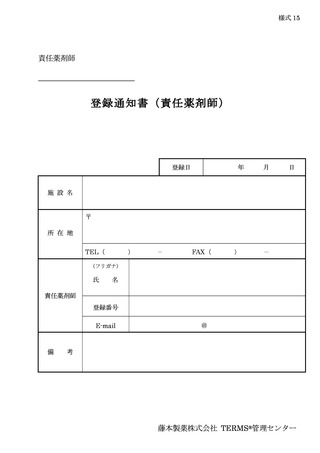
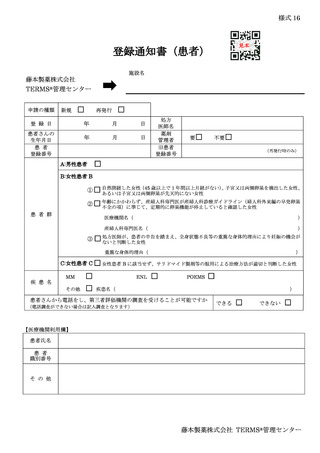
6.3.2.登録通知 ········································································ 12
6.4.登録情報 ············································································
12
6.4.1.藤本製薬株式会社登録情報 ··············································
12
6.4.2.医療機関登録情報 ···························································· 13
6.5.登録申請内容の確認 ······························································ 13
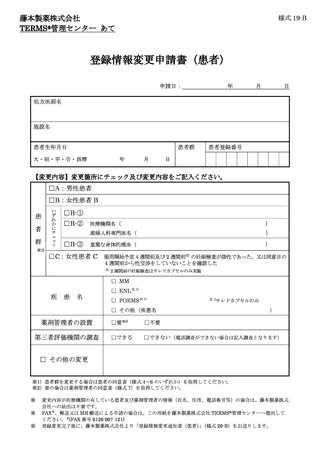
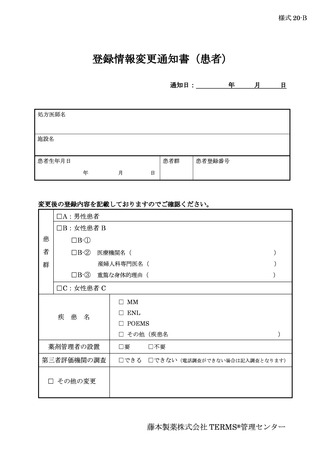
6.6.登録情報の変更 ···································································
13
7.流通、処方及び調剤 ··································································
15
7.1.流通 ·················································································
15
7.2.処方 ·················································································
15
7.3.調剤 ·················································································
16
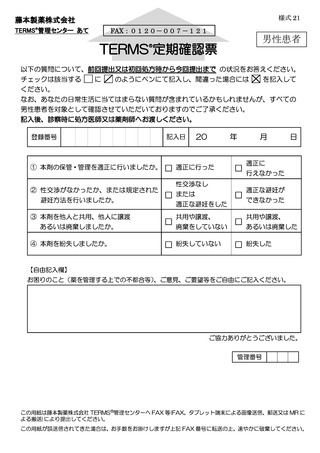
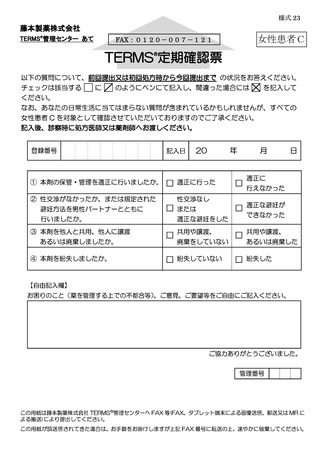
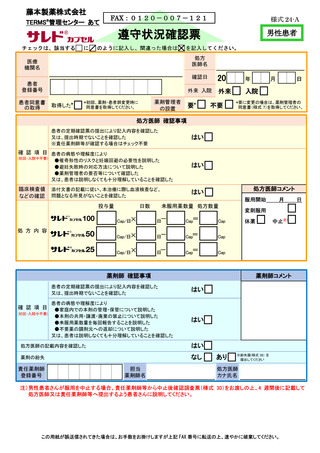
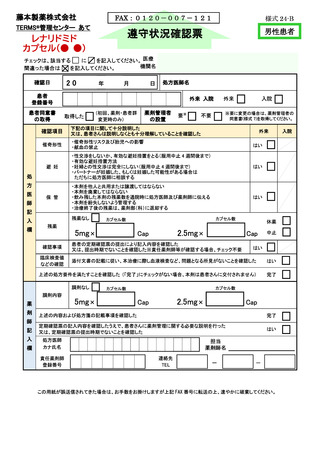
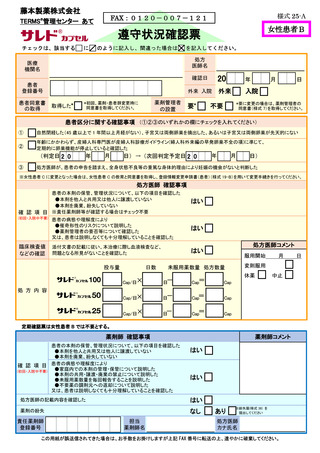
7.4.遵守状況の定期確認 ···························································
17
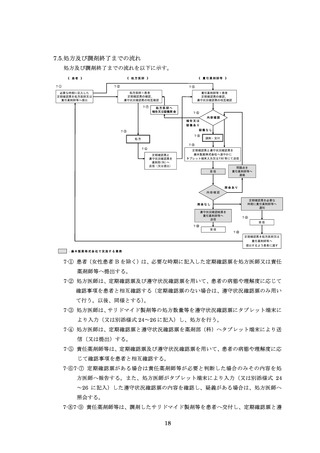
7.5.処方及び調剤終了までの流れ ···············································
18
7.6.本手順の運用状況の確認 ·····················································
19
8.薬剤管理及び妊娠回避の徹底等 ··················································
20
8.1.薬剤管理 ··········································································
20
8.1.1.保管場所 ·····································································
20
8.1.2.数量管理 ·····································································
20
8.1.2.1.医療機関及び特約店の数量管理 ·································
20
8.1.2.2.患者の数量管理 ······················································
20
8.1.2.3.入院中の数量管理 ···················································
20
目次1
1.背景 ··························································································
1
2.目的 ··························································································
1
3.用語の定義 ·················································································
2
4.関連組織 ····················································································
4
4.1.医療機関 ·············································································
4
4.2.特約店·················································································
4
4.3.TERMS 委員会 ·····································································
4
4.4.第三者評価機関 ····································································
4
4.5.組織図·················································································
4
5.情報提供及び教育 ········································································
5
5.1.対象者·················································································
5
5.2.実施方法 ·············································································
5
6.登録 ··························································································
9
6.1.登録対象者 ··········································································
9
6.2.登録要件 ·············································································
9
6.3.登録手順·············································································
11
6.3.1.登録申請 ········································································ 11
6.3.2.登録通知 ········································································ 12
6.4.登録情報 ············································································
12
6.4.1.藤本製薬株式会社登録情報 ··············································
12
6.4.2.医療機関登録情報 ···························································· 13
6.5.登録申請内容の確認 ······························································ 13
6.6.登録情報の変更 ···································································
13
7.流通、処方及び調剤 ··································································
15
7.1.流通 ·················································································
15
7.2.処方 ·················································································
15
7.3.調剤 ·················································································
16
7.4.遵守状況の定期確認 ···························································
17
7.5.処方及び調剤終了までの流れ ···············································
18
7.6.本手順の運用状況の確認 ·····················································
19
8.薬剤管理及び妊娠回避の徹底等 ··················································
20
8.1.薬剤管理 ··········································································
20
8.1.1.保管場所 ·····································································
20
8.1.2.数量管理 ·····································································
20
8.1.2.1.医療機関及び特約店の数量管理 ·································
20
8.1.2.2.患者の数量管理 ······················································
20
8.1.2.3.入院中の数量管理 ···················································
20
目次1