よむ、つかう、まなぶ。
参考資料1-2 TERMS®サリドマイド製剤等安全管理手順[6.2MB] (3 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_40908.html |
| 出典情報 | 薬事審議会 医薬品等安全対策部会安全対策調査会(令和6年度第3回 6/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
8.1.2.4.未服用薬の数量管理 ················································
21
8.1.3.カプセルシート、薬剤管理キット ···································
21
8.1.4.薬剤の返却 ··································································
21
8.1.5.薬剤の廃棄 ··································································
21
8.1.6.薬剤紛失時の対応 ·························································
21
8.1.6.1.医療機関又は特約店における紛失 ······························
21
8.1.6.2.患者による紛失 ······················································
22
8.2.妊娠回避の徹底 ·································································
22
8.2.1.対象者 ········································································
22
8.2.2.妊娠回避の期間 ····························································
22
8.2.3.妊娠回避の方法 ····························································
22
8.3.諸検査の実施(妊娠検査、血液検査等) ································
23
8.3.1.妊娠検査 ·····································································
23
8.3.2.血液検査等 ··································································
24
8.4.禁止事項···········································································
24
8.4.1.禁止項目及び禁止期間 ···················································
24
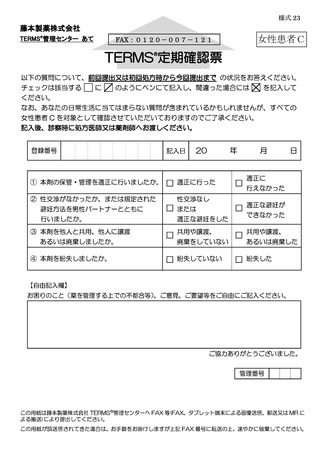
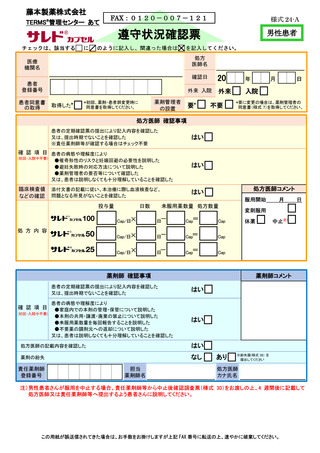
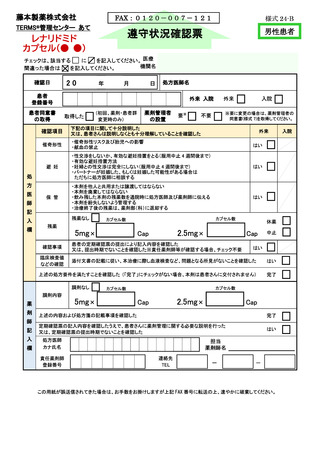
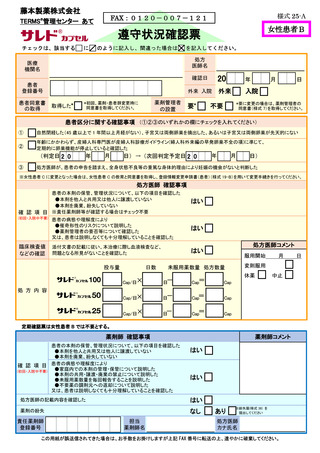
8.4.2.禁止項目の遵守状況確認················································
24
8.5.逸脱時の対応 ····································································
25
9.評価及び改善 ···········································································
26
9.1.評価 ·················································································
26
9.1.1.TERMS 委員会による評価 ·············································
26
9.1.2.第三者評価機関による評価 ··········································
27
9.2.改善 ·················································································
27
10.その他 ··················································································
28
10.1.情報の公開 ······································································
28
10.1.1.本手順の公開 ·····························································
28
10.1.2.遵守状況等の公開 ·······················································
28
10.2.行政への報告 ···································································
28
10.2.1.定期報告 ···································································
28
10.2.2.緊急報告 ···································································
29
10.2.3.追跡調査報告 ·····························································
29
10.3.記録の保存 ······································································
29
10.4.情報の管理及び個人情報の保護 ··········································
29
10.4.1.情報の管理 ································································
29
10.4.2.個人情報の保護 ··························································
30
10.5.適応外使用 ······································································
30
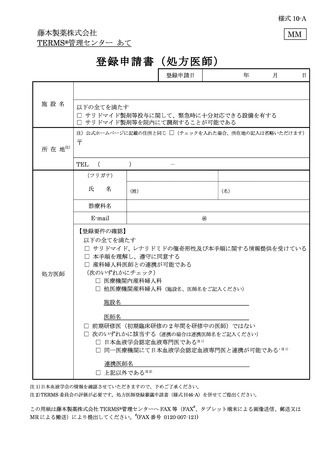
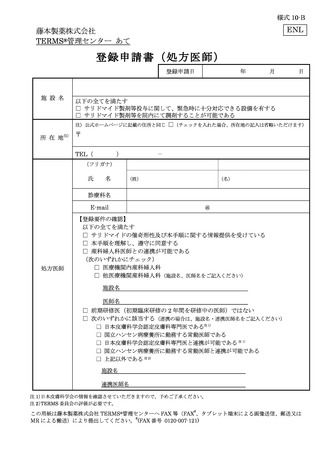
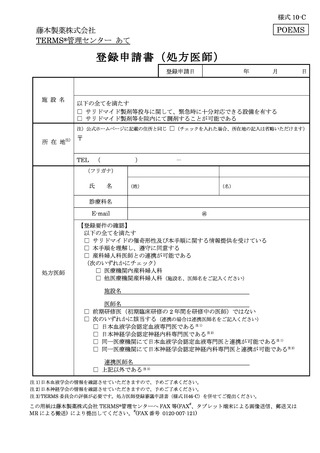
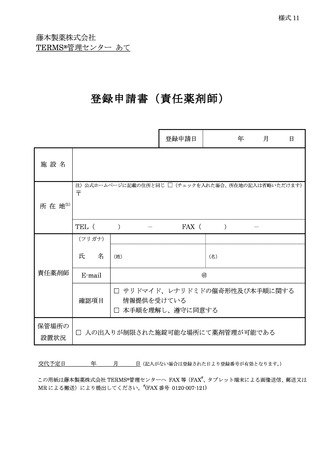
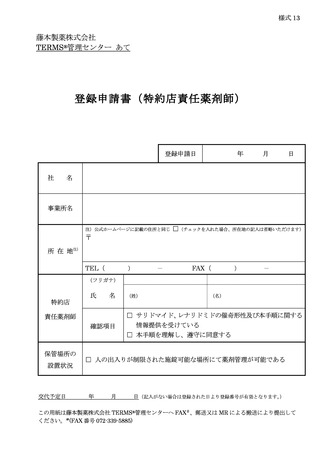
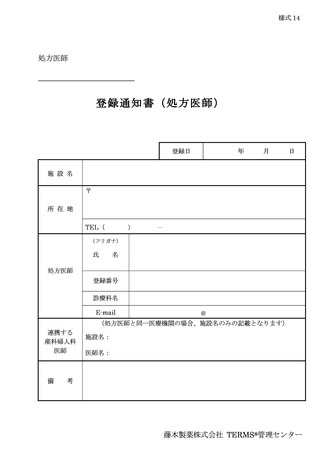
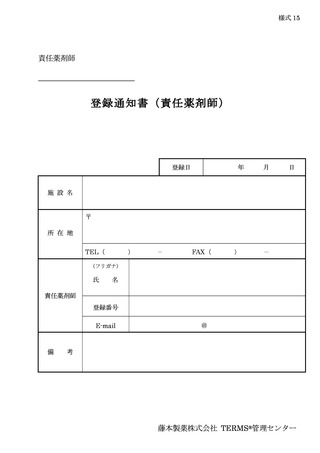
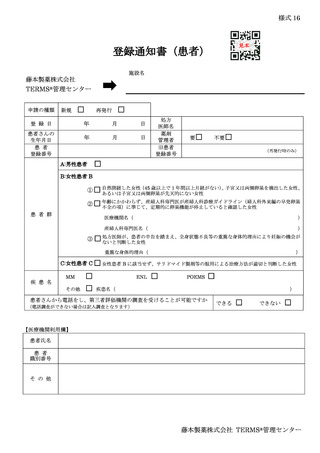
10.6.主な様式 ·········································································
30
目次2
21
8.1.3.カプセルシート、薬剤管理キット ···································
21
8.1.4.薬剤の返却 ··································································
21
8.1.5.薬剤の廃棄 ··································································
21
8.1.6.薬剤紛失時の対応 ·························································
21
8.1.6.1.医療機関又は特約店における紛失 ······························
21
8.1.6.2.患者による紛失 ······················································
22
8.2.妊娠回避の徹底 ·································································
22
8.2.1.対象者 ········································································
22
8.2.2.妊娠回避の期間 ····························································
22
8.2.3.妊娠回避の方法 ····························································
22
8.3.諸検査の実施(妊娠検査、血液検査等) ································
23
8.3.1.妊娠検査 ·····································································
23
8.3.2.血液検査等 ··································································
24
8.4.禁止事項···········································································
24
8.4.1.禁止項目及び禁止期間 ···················································
24
8.4.2.禁止項目の遵守状況確認················································
24
8.5.逸脱時の対応 ····································································
25
9.評価及び改善 ···········································································
26
9.1.評価 ·················································································
26
9.1.1.TERMS 委員会による評価 ·············································
26
9.1.2.第三者評価機関による評価 ··········································
27
9.2.改善 ·················································································
27
10.その他 ··················································································
28
10.1.情報の公開 ······································································
28
10.1.1.本手順の公開 ·····························································
28
10.1.2.遵守状況等の公開 ·······················································
28
10.2.行政への報告 ···································································
28
10.2.1.定期報告 ···································································
28
10.2.2.緊急報告 ···································································
29
10.2.3.追跡調査報告 ·····························································
29
10.3.記録の保存 ······································································
29
10.4.情報の管理及び個人情報の保護 ··········································
29
10.4.1.情報の管理 ································································
29
10.4.2.個人情報の保護 ··························································
30
10.5.適応外使用 ······································································
30
10.6.主な様式 ·········································································
30
目次2