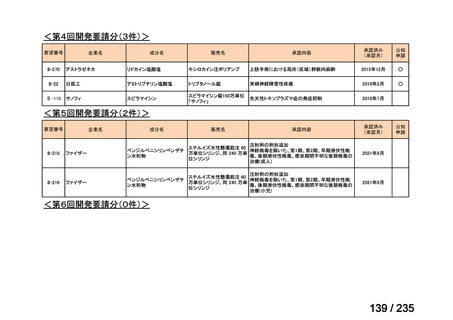
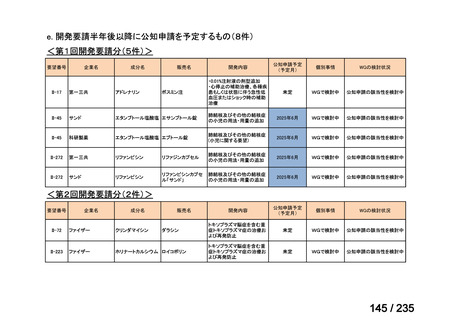
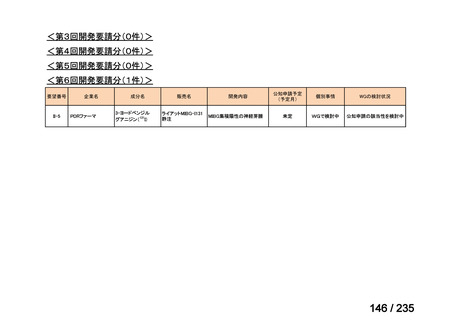
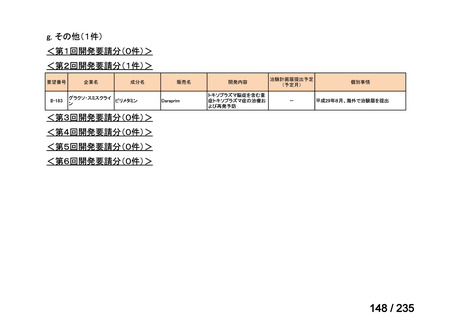
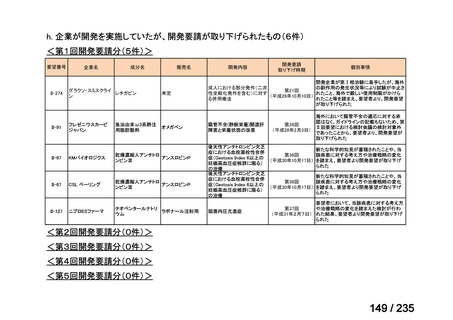
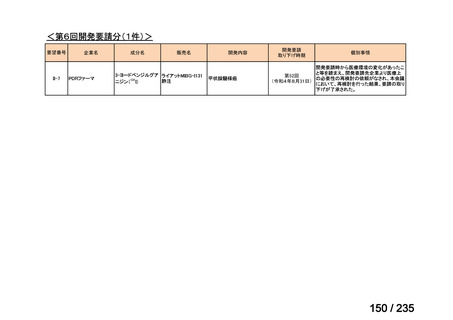
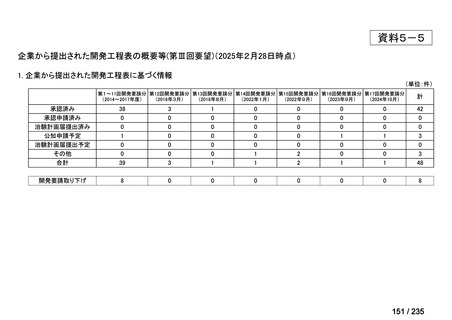
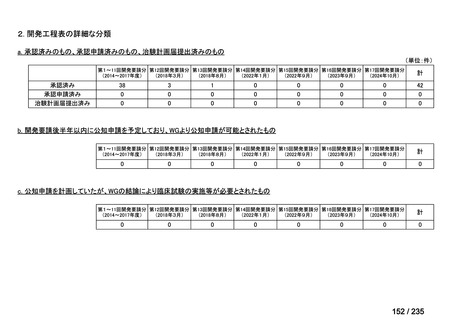
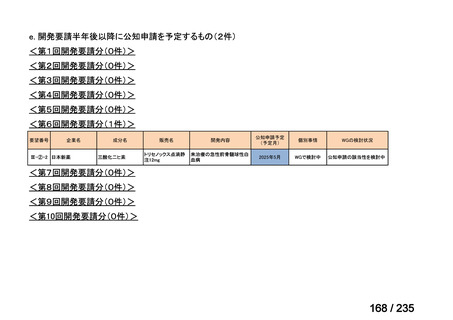
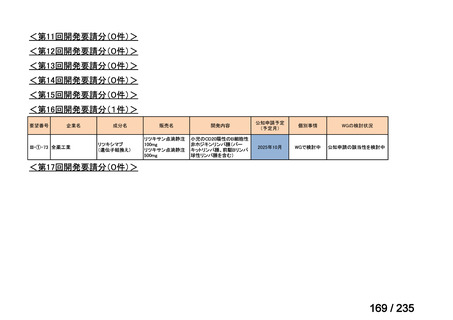
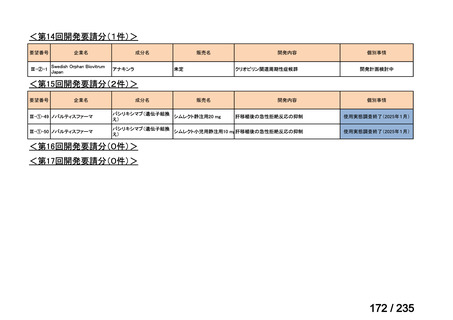
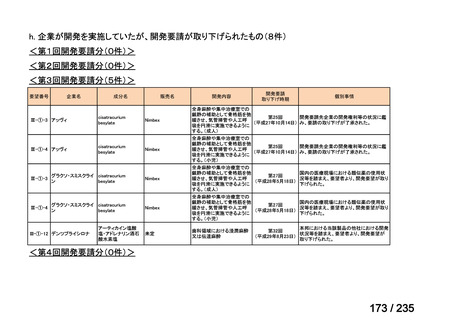
会議資料 (85 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00036.html |
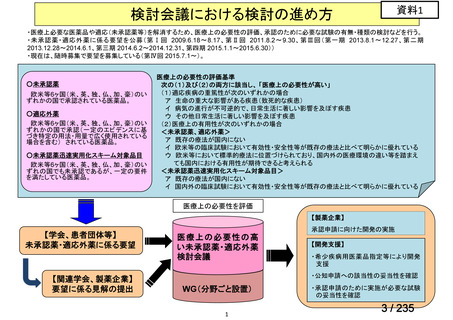
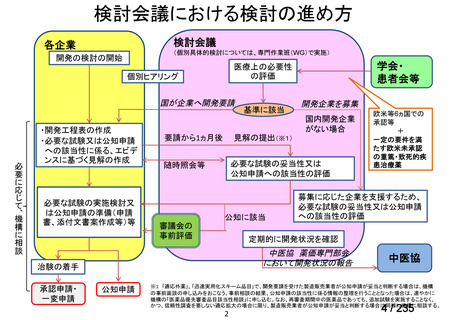
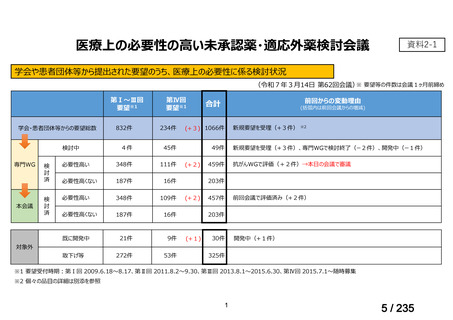
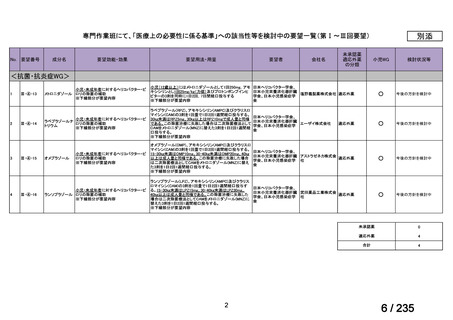
| 出典情報 | 医薬・生活衛生局が実施する検討会 医療上の必要性の高い未承認薬・適応外薬検討会議(第62回 3/13)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
5.
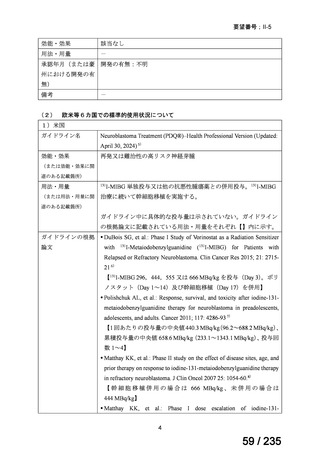
PDQ® Pediatric Treatment Editorial Board. PDQ Neuroblastoma Treatment. Bethesda, MD:
National Cancer Institute. Updated Apr 30, 2024. Available at:
https://www.cancer.gov/types/neuroblastoma/hp/neuroblastoma-treatment-pdq. Accessed Aug
19, 2024.
6.
DuBois SG, Groshen S, Park JR, Haas-Kogan DA, Yang X, Geier E, et al. Phase I Study of
Vorinostat as a Radiation Sensitizer with 131I-Metaiodobenzylguanidine (131I-MIBG) for
Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res 2015; 21: 2715-21.
7.
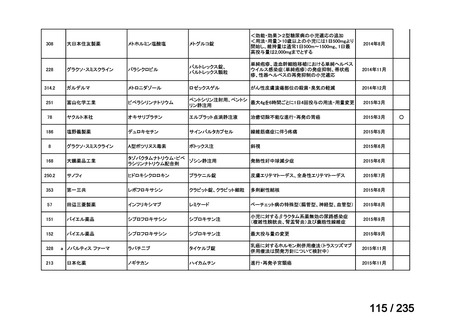
Polishchuk AL, Dubois SG, Haas-Kogan D, Hawkins R, Matthay KK. Response, survival, and
toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in
preadolescents, adolescents, and adults. Cancer 2011; 117: 4286-93.
8.
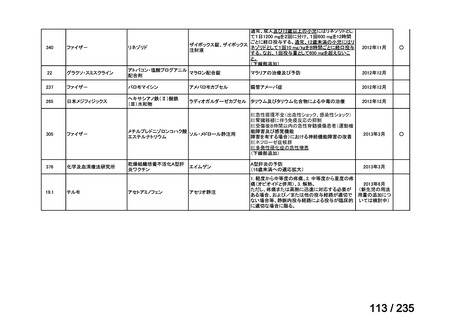
Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the
effect of disease sites, age, and prior therapy on response to iodine-131metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 2007; 25: 105460.
9.
Matthay KK, Tan JC, Villablanca JG, Yanik GA, Veatch J, Franc B, et al. Phase I dose
escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and
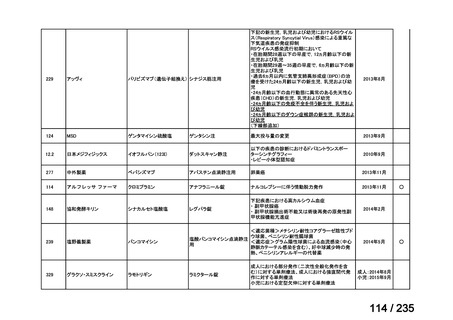
autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to
Neuroblastoma Therapy Consortium Study. J Clin Oncol 2006; 24: 500-6.
10. Matthay KK, Quach A, Huberty J, Franc BL, Hawkins RA, Jackson H, et al. Iodine-131metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma:
a new approaches to neuroblastoma therapy phase I study. J Clin Oncol 2009; 27: 1020-5.
11. DuBois SG, Chesler L, Groshen S, Hawkins R, Goodarzian F, Shimada H, et al. Phase I study
of vincristine, irinotecan, and ¹³¹I-metaiodobenzylguanidine for patients with relapsed or
refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res
2012; 18: 2679-86.
12. Johnson K, McGlynn B, Saggio J, Baniewicz D, Zhuang H, Maris JM, et al. Safety and
efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory
neuroblastoma. Pediatr Blood Cancer 2011; 57: 1124-9.
13. French S, DuBois SG, Horn B, Granger M, Hawkins R, Pass A, et al. 131I-MIBG followed by
consolidation with busulfan, melphalan and autologous stem cell transplantation for refractory
neuroblastoma. Pediatr Blood Cancer 2013; 60: 879-84.
14. Zhou MJ, Doral MY, DuBois SG, Villablanca JG, Yanik GA, Matthay KK. Different outcomes
for relapsed versus refractory neuroblastoma after therapy with 131I-metaiodobenzylguanidine
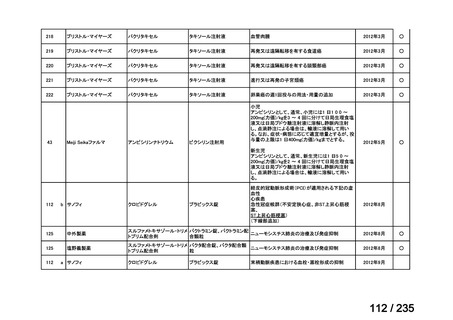
(131I-MIBG). Eur J Cancer 2015; 51: 2465-72.
15. Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A, et al. 131Imetaiodobenzylguanidine with intensive chemotherapy and autologous stem cell
transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy
30
85 / 235