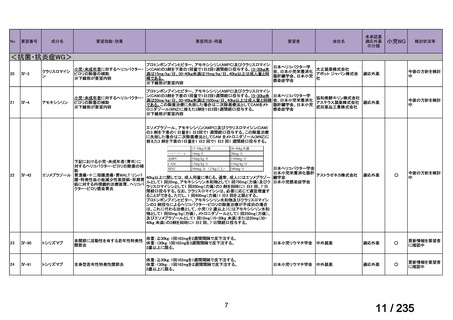
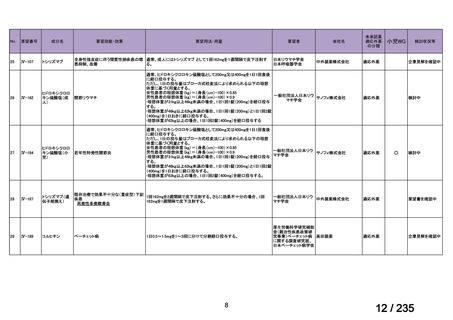
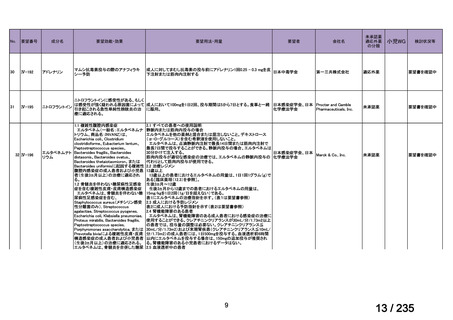
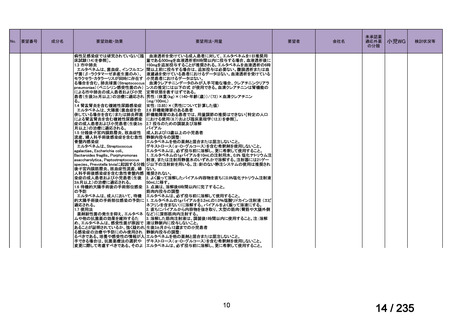
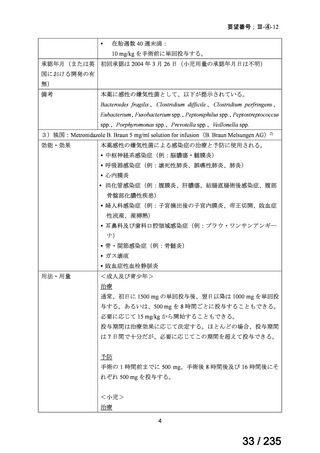
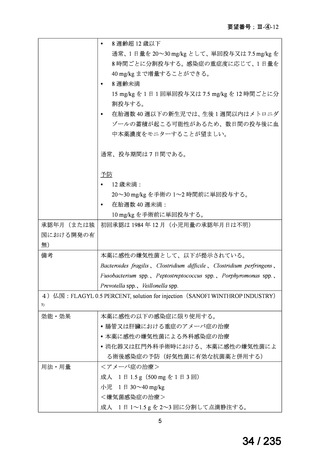
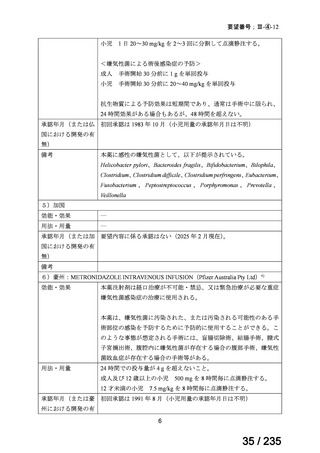
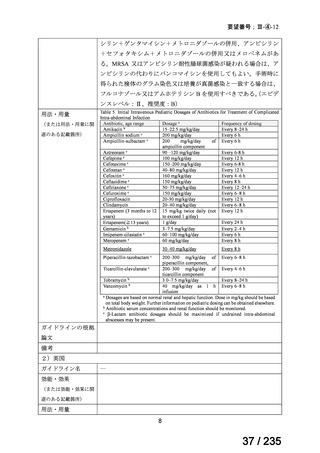
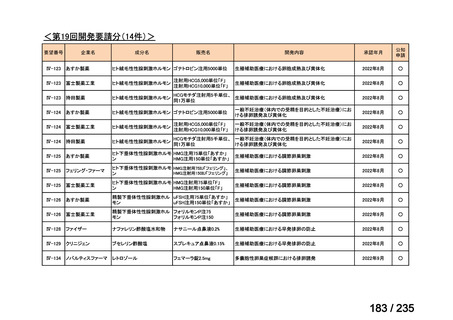
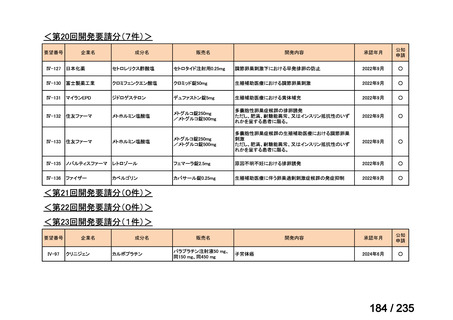
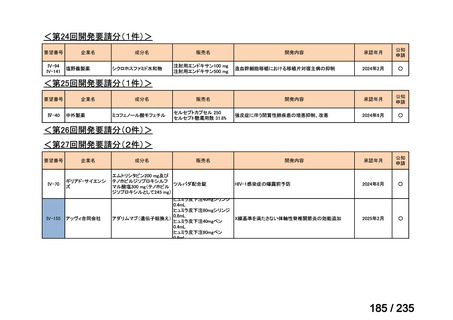
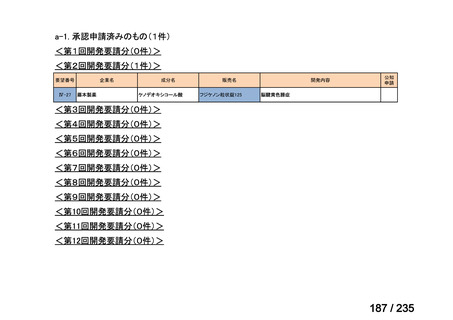
会議資料 (86 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00036.html |
| 出典情報 | 医薬・生活衛生局が実施する検討会 医療上の必要性の高い未承認薬・適応外薬検討会議(第62回 3/13)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
(NANT) phase II study. Biol Blood Marrow Transplant 2015; 21: 673-81.
16. DuBois SG, Granger MM, Groshen S, Tsao-Wei D, Ji L, Shamirian A, et al. Randomized Phase
II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for
Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium. J
Clin Oncol 2021; 39: 3506-14.
17. Sevrin F, et al. Phase II study of (131) I-metaiodobenzylguanidine with 5 days of topotecan for
refractory or relapsed neuroblastoma: Results of the French study MIITOP. Pediatr Blood
Cancer 2023; 70: e30615.
18. Children's Cancer and Leukaemia Group. Options fo the Treatment of Patients with
Relapsed/Progressive High-Risk Neuroblastoma: British Associstion of Paediatric Surgeons,;
2015 [cited 2021 Jul]. Available from:
https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/CCL
G_Relapsed_Progressive_High_Risk_Neuroblastoma_Guidelines_March_2015_FINAL.pdf.
19. Gaze MN, Chang YC, Flux GD, Mairs RJ, Saran FH, Meller ST. Feasibility of dosimetry-based
high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with
metastatic neuroblastoma. Cancer Biother Radiopharm 2005; 20: 195-9.
20. Giammarile F, Chiti A, Lassmann M, Brans B, Flux G. EANM procedure guidelines for 131Imeta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 2008; 35: 103947.
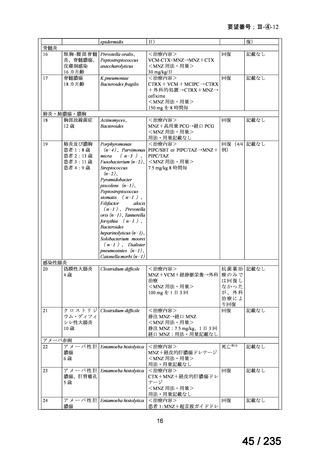
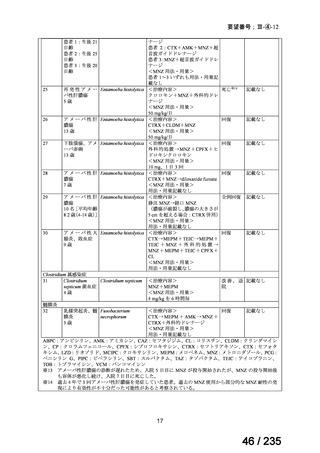
21. Lashford LS, Lewis IJ, Fielding SL, Flower MA, Meller S, Kemshead JT, et al. Phase I/II study
of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: a United Kingdom
Children's Cancer Study Group investigation. J Clin Oncol 1992; 10: 1889-96.
22. Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, et al. Correlation of tumor
and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma
treated with 131I-MIBG. J Nucl Med 2001; 42: 1713-21.
23. Simon T, Hero B, Schulte JH, Deubzer H, Hundsdoerfer P, von Schweinitz D, et al. 2017
GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klin
Padiatr 2017; 229: 147-67.
24. Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose
escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory
neuroblastoma. J Clin Oncol 1998; 16: 229-36.
25. Matthay KK, Weiss B, Villablanca JG, Maris JM, Yanik GA, Dubois SG, et al. Dose escalation
study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory
neuroblastoma: new approaches to neuroblastoma therapy consortium trial. J Nucl Med 2012;
53: 1155-63.
26. de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients
31
86 / 235