【資料No.1】2.5_臨床に関する概括資料 (160 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29325.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第5回 11/22)、医薬品第二部会(令和4年度第13回 11/22)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
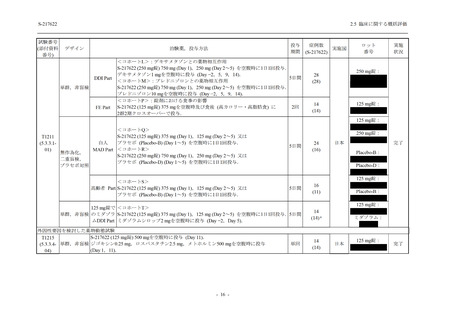
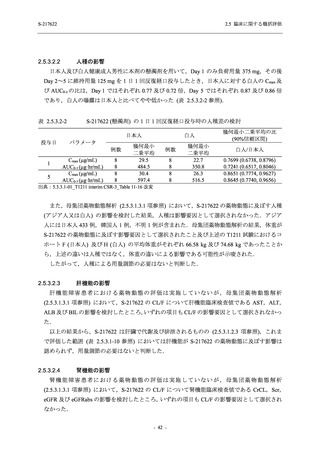
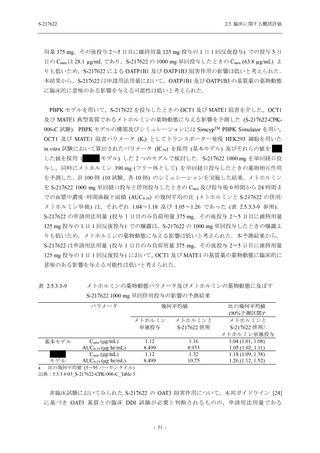
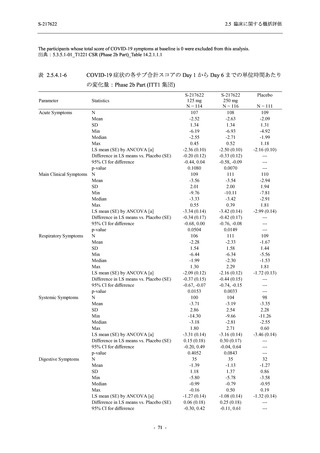
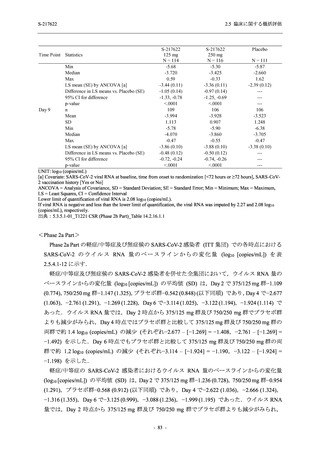
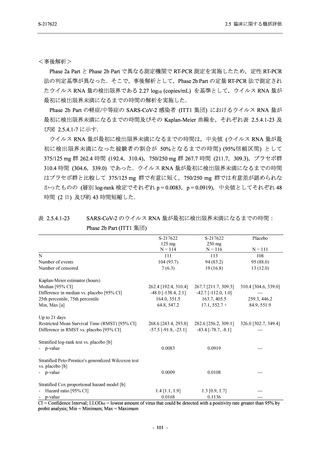
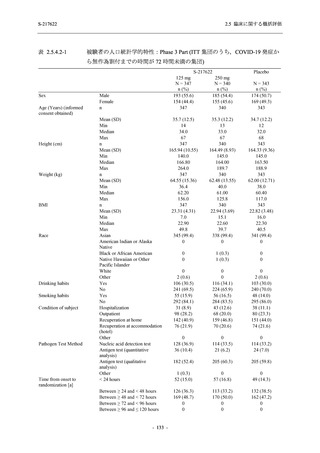
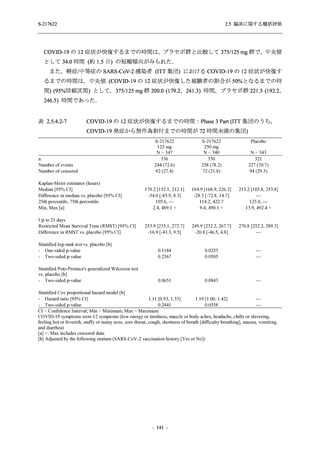
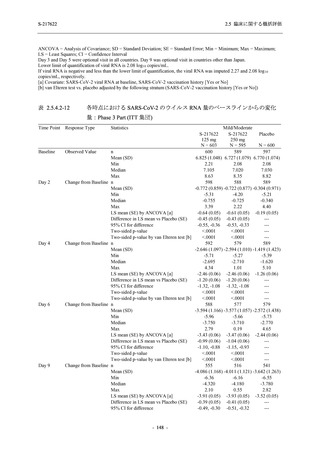
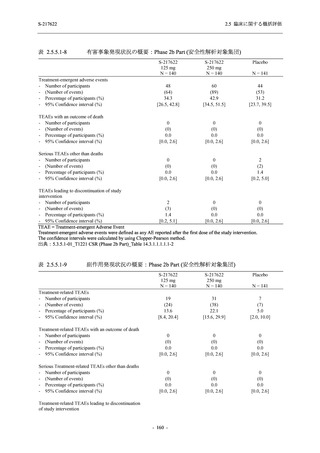
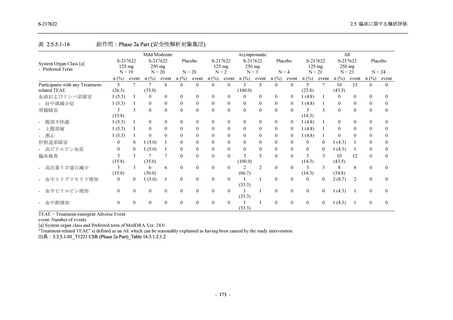
表 2.5.5.1-8
2.5 臨床に関する概括評価
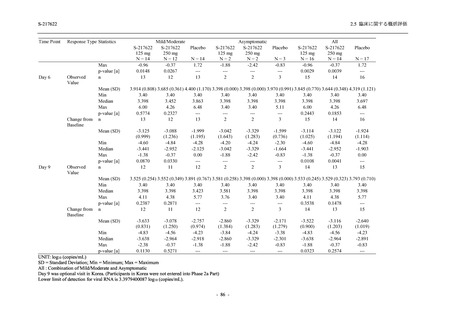
有害事象発現状況の概要:Phase 2b Part (安全性解析対象集団)
S-217622
125 mg
N = 140
S-217622
250 mg
N = 140
N = 141
48
(64)
34.3
[26.5, 42.8]
60
(89)
42.9
[34.5, 51.5]
44
(53)
31.2
[23.7, 39.5]
TEAEs with an outcome of death
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
Serious TEAEs other than deaths
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
2
(2)
1.4
[0.2, 5.0]
Treatment-emergent adverse events
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
Placebo
TEAEs leading to discontinuation of study
intervention
- Number of participants
2
0
0
- (Number of events)
(3)
(0)
(0)
- Percentage of participants (%)
1.4
0.0
0.0
- 95% Confidence interval (%)
[0.2, 5.1]
[0.0, 2.6]
[0.0, 2.6]
TEAE = Treatment-emergent Adverse Event
Treatment-emergent adverse events were defined as any AE reported after the first dose of the study intervention.
The confidence intervals were calculated by using Clopper-Pearson method.
出典:5.3.5.1-01_T1221 CSR (Phase 2b Part)_Table 14.3.1.1.1.1.1-2
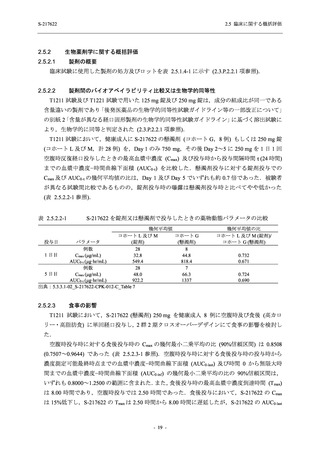
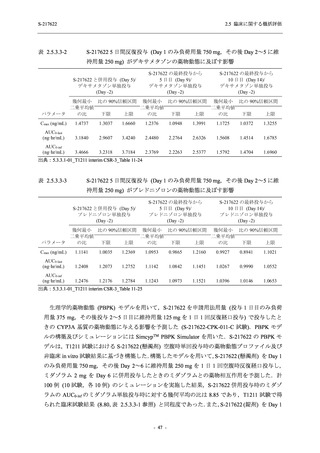
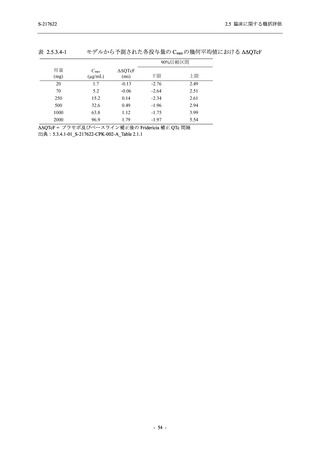
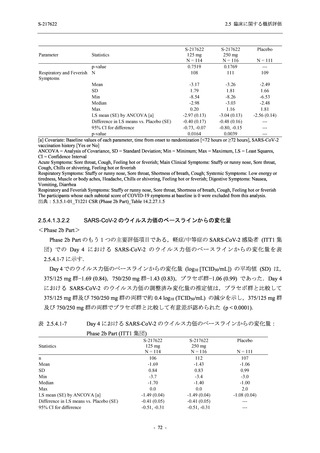
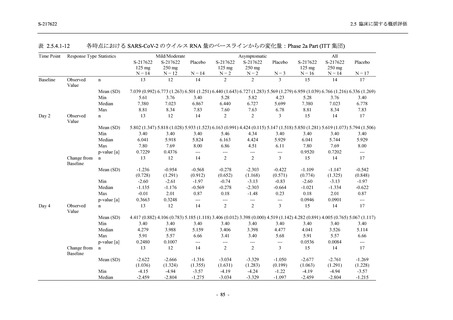
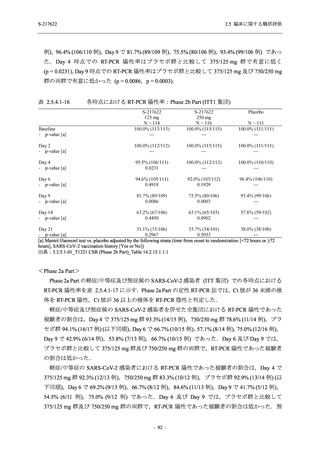
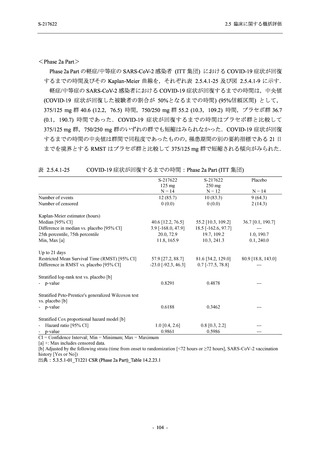
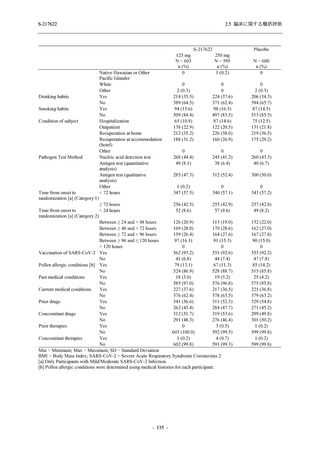
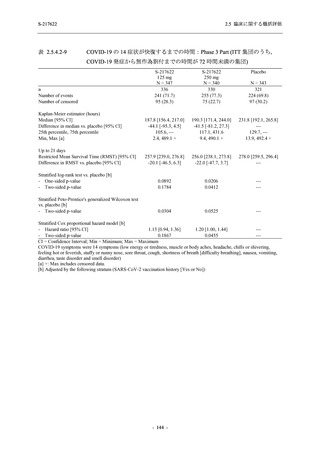
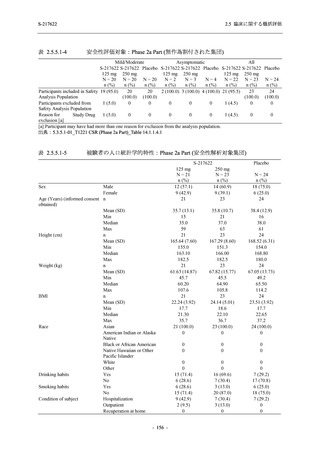
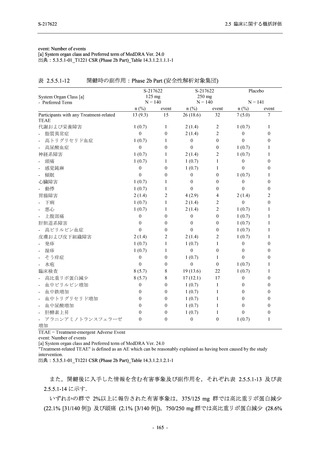
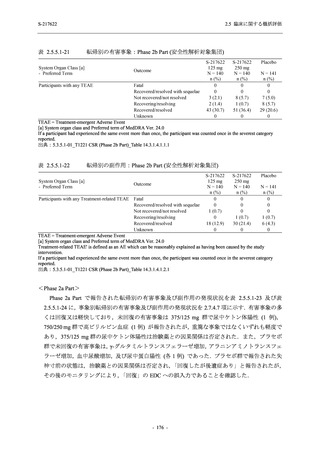
表 2.5.5.1-9
副作用発現状況の概要:Phase 2b Part (安全性解析対象集団)
S-217622
125 mg
N = 140
S-217622
250 mg
N = 140
N = 141
Treatment-related TEAEs
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
19
(24)
13.6
[8.4, 20.4]
31
(38)
22.1
[15.6, 29.9]
7
(7)
5.0
[2.0, 10.0]
Treatment-related TEAEs with an outcome of death
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
Serious Treatment-related TEAEs other than deaths
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
0
(0)
0.0
[0.0, 2.6]
Treatment-related TEAEs leading to discontinuation
of study intervention
- 160 -
Placebo