【資料No.1】2.5_臨床に関する概括資料 (177 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29325.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第5回 11/22)、医薬品第二部会(令和4年度第13回 11/22)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
2.5 臨床に関する概括評価
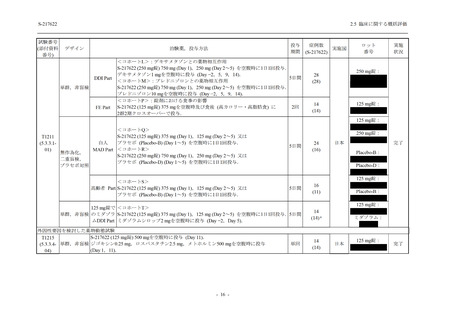
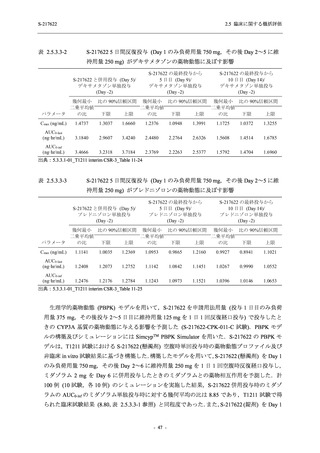
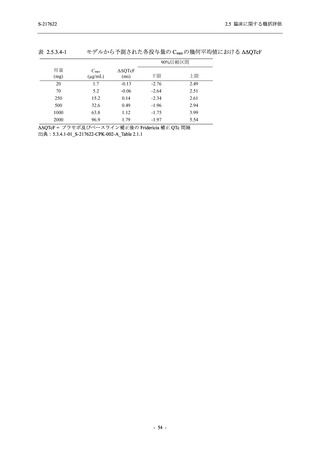
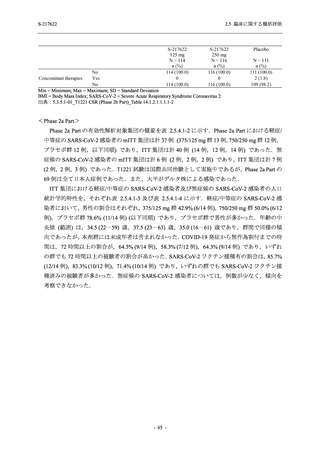
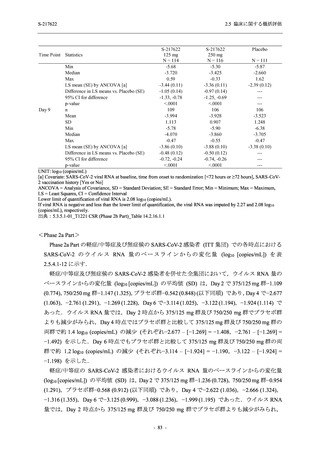
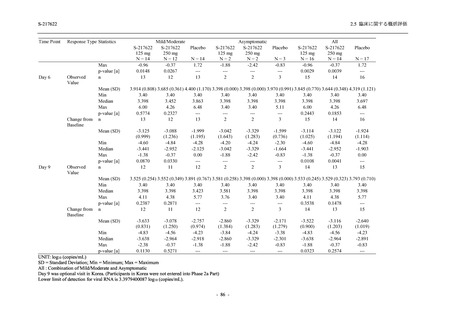
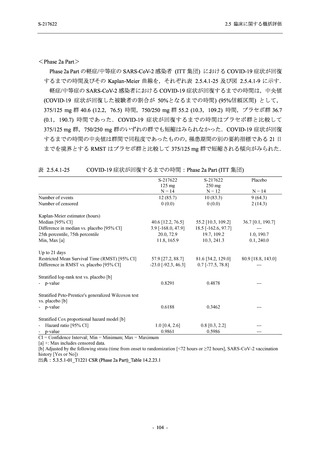
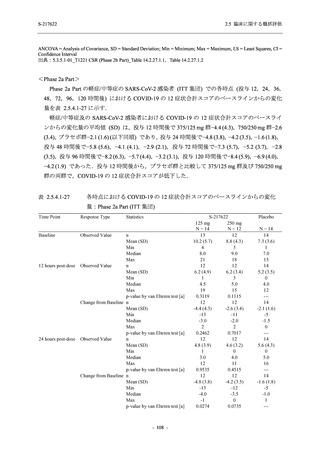
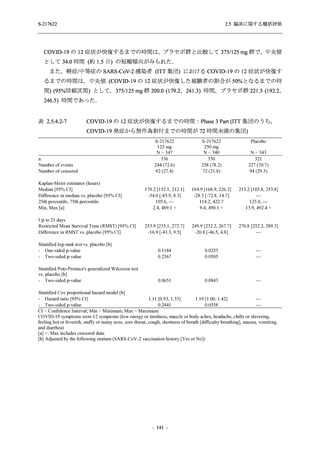
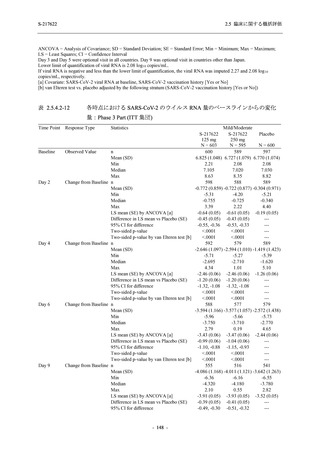
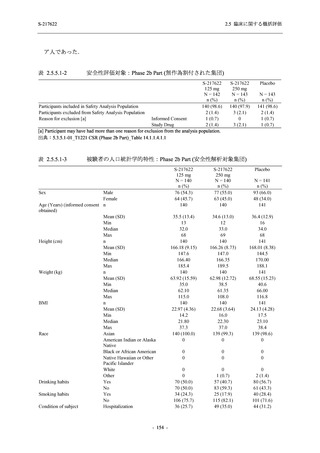
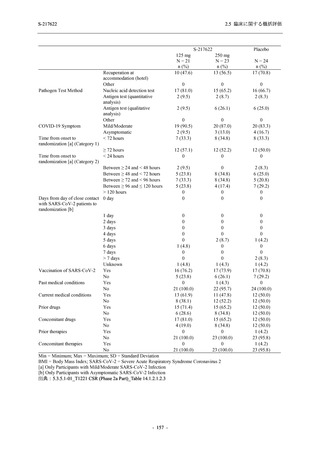
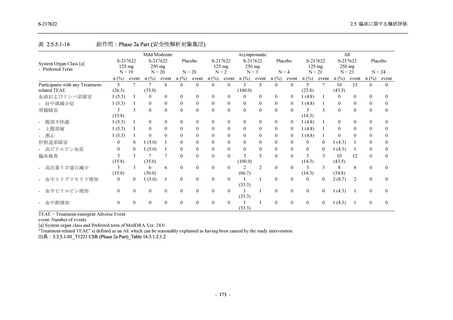
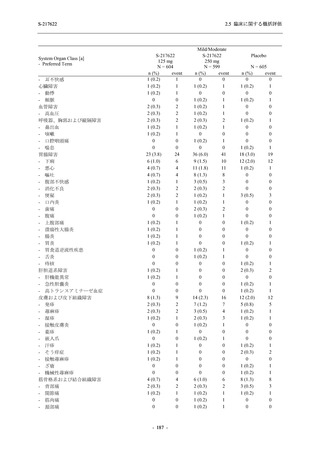
表 2.5.5.1-23
転帰別の有害事象:Phase 2a Part (安全性解析対象集団)
System Organ
Class [a]
Outcome
- Preferred Term
Participants with
any TEAE
Fatal
Mild/Moderate
Asymptomatic
All
S-217622 S-217622 Placebo S-217622 S-217622 Placebo S-217622 S-217622 Placebo
125 mg 250 mg
125 mg 250 mg
125 mg 250 mg
N = 19 N = 20 N = 20 N = 2
N=3
N = 4 N = 21 N = 23 N = 24
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
0
0
0
0
0
0
0
0
0
Recovered/
0
0
1 (5.0)
0
0
0
0
0
1 (4.2)
resolved with
sequelae
Not recovered/ 1 (5.3) 1 (5.0) 2 (10.0)
0
0
0
1 (4.8) 1 (4.3) 2 (8.3)
not resolved
Recovering/
1 (5.3)
0
1 (5.0)
0
0
0
1 (4.8)
0
1 (4.2)
resolving
Recovered/
8 (42.1) 12 (60.0) 5 (25.0) 1 (50.0) 3 (100.0)
0
9 (42.9) 15 (65.2) 5 (20.8)
resolved
Unknown
0
0
0
0
0
0
0
0
0
TEAE = Treatment-emergent Adverse Event
[a] System organ class and Preferred term of MedDRA Ver. 24.0
If a participant had experienced the same event more than once, the participant was counted once in the worst outcome
category reported.
出典:5.3.5.1-01_T1221 CSR (Phase 2a Part)_Table 14.3.1.4.1.1
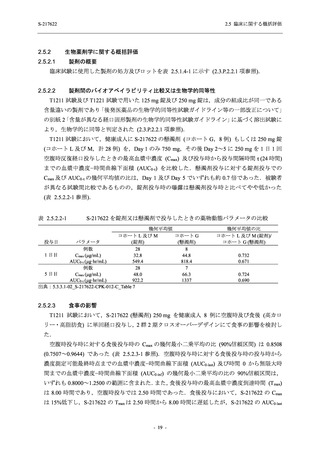
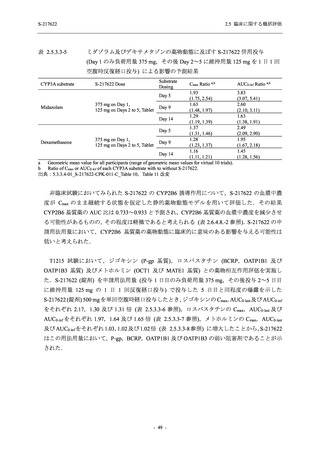
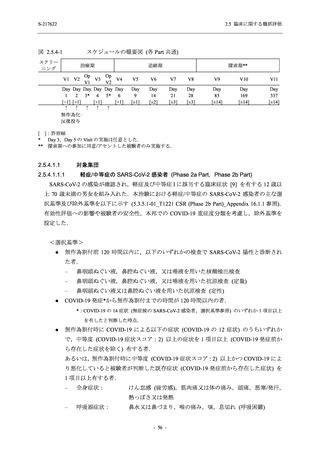
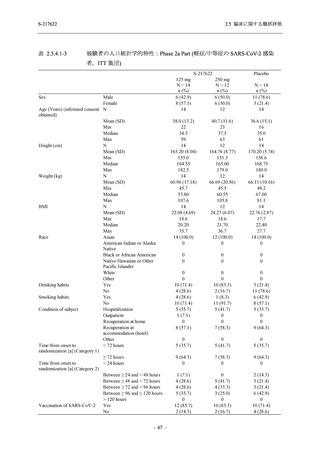
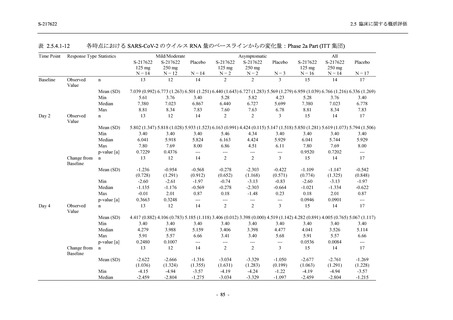
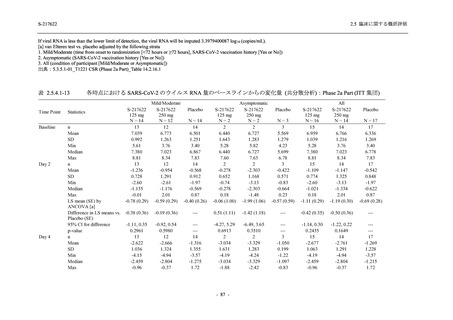
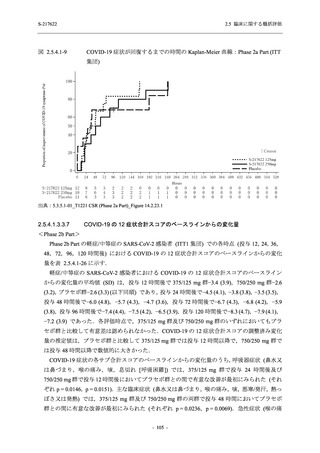
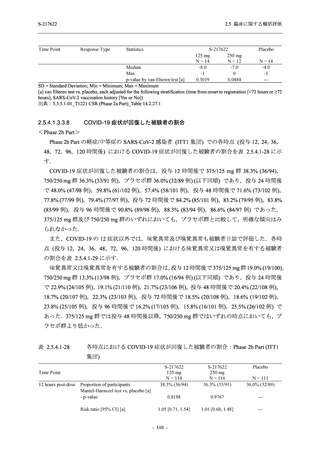
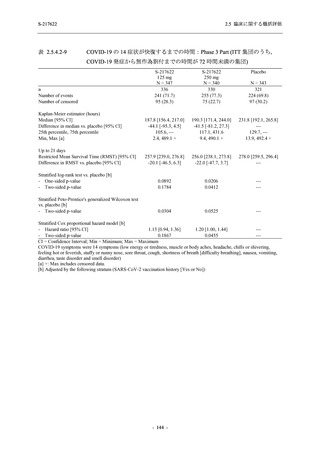
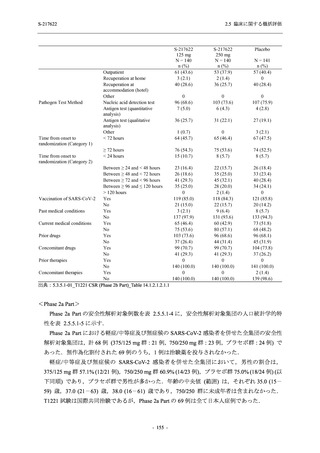
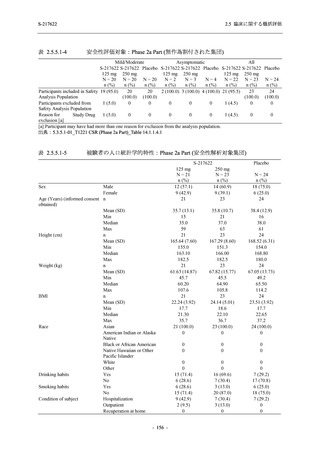
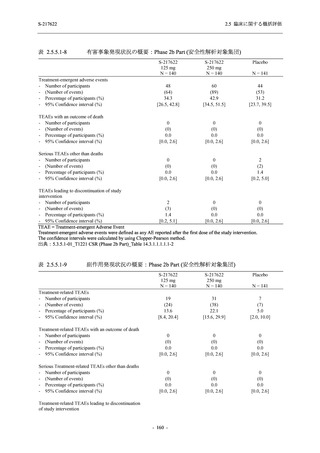
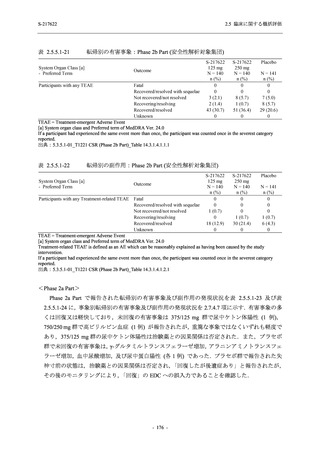
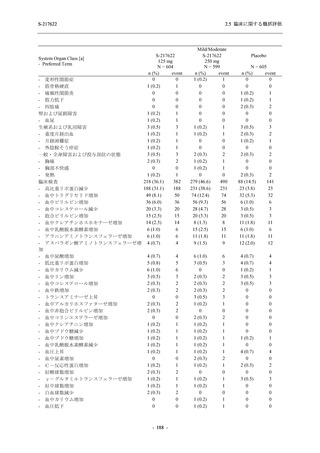
表 2.5.5.1-24
転帰別の副作用:Phase 2a Part (安全性解析対象集団)
System Organ
Class [a]
Outcome
- Preferred Term
Participants with
any Treatmentrelated TEAE
Fatal
Mild/Moderate
Asymptomatic
All
S-217622 S-217622 Placebo S-217622 S-217622 Placebo S-217622 S-217622 Placebo
125 mg 250 mg
125 mg 250 mg
125 mg 250 mg
N = 19 N = 20 N = 20 N = 2
N=3
N = 4 N = 21 N = 23 N = 24
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
0
0
0
0
0
0
0
0
0
Recovered/
0
0
0
0
0
0
0
0
resolved with
sequelae
Not recovered/
0
1 (5.0)
0
0
0
0
0
1 (4.3)
not resolved
Recovering/
0
0
0
0
0
0
0
0
resolving
Recovered/
5 (26.3) 6 (30.0)
0
0
3 (100.0)
0
5 (23.8) 9 (39.1)
resolved
Unknown
0
0
0
0
0
0
0
0
TEAE = Treatment-emergent Adverse Event
[a] System organ class and Preferred term of MedDRA Ver. 24.0
"Treatment-related TEAE" is defined as an AE which can be reasonably explained as having been caused by the study
intervention.
If a participant had experienced the same event more than once, the participant was counted once in the worst outcome
category reported.
出典:5.3.5.1-01_T1221 CSR (Phase 2a Part)_Table 14.3.1.4.1.2
2.5.5.1.2.1.4
0
0
0
0
0
発現時期別
<Phase 2b Part>
開鍵後に入手した情報を含む発現時期別の有害事象及び副作用の発現状況を表 2.5.5.1-25 及
び表 2.5.5.1-26 に,事象別発現時期別の有害事象及び副作用の発現状況を 2.7.4.7 項に示す.プ
ラセボ群と比較して,本剤群で Day 6~7 に認められた有害事象が多かったが,臨床検査値の測
- 177 -