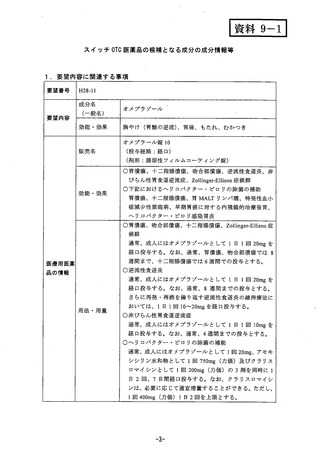
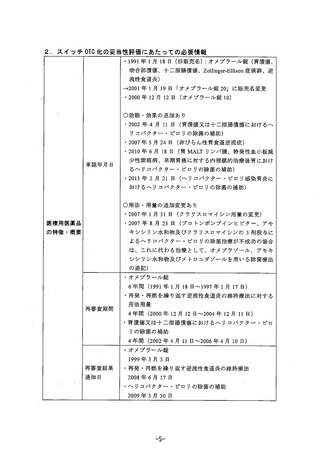
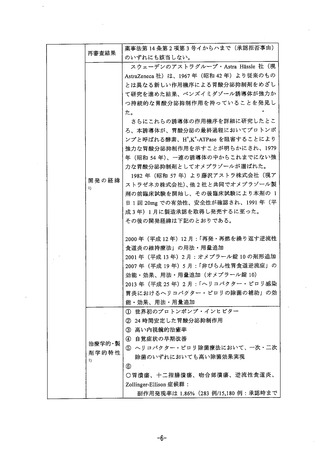
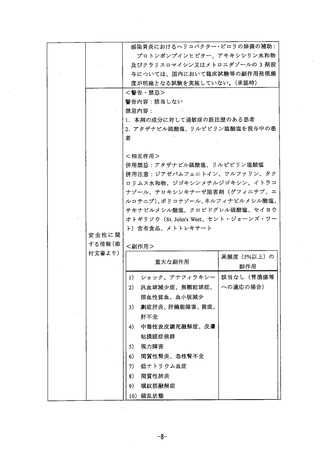
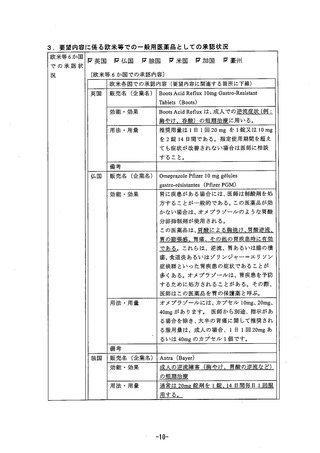
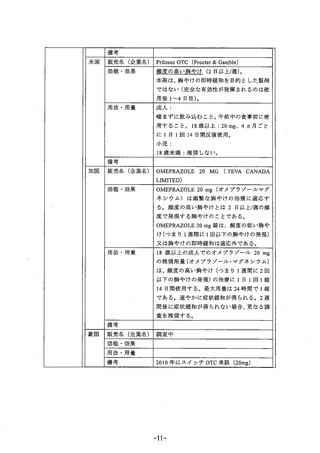
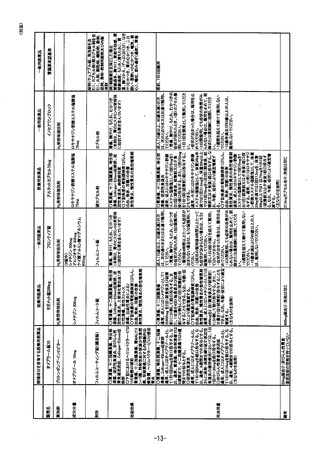
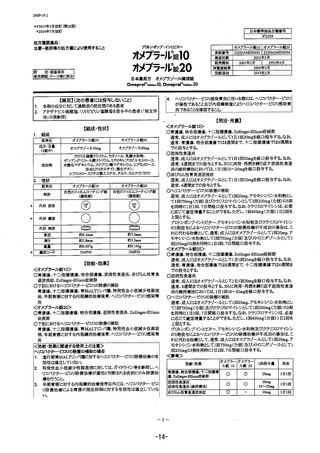
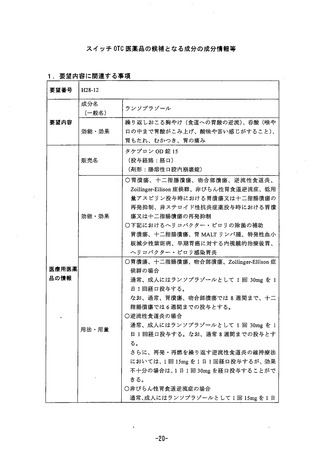
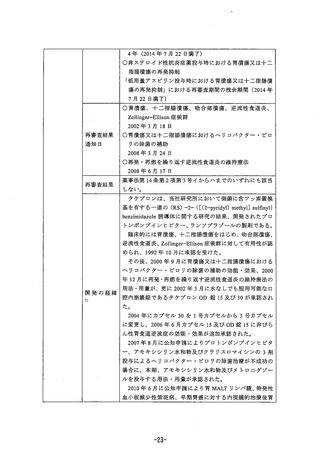
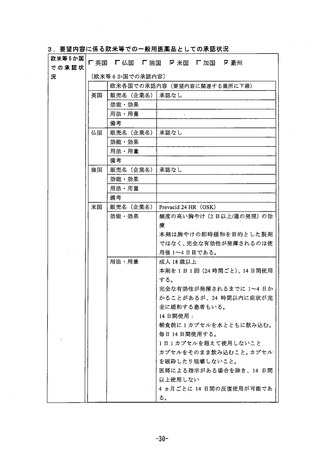
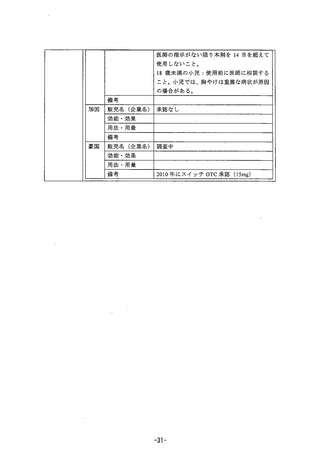
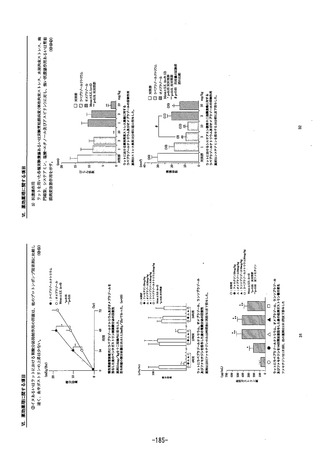
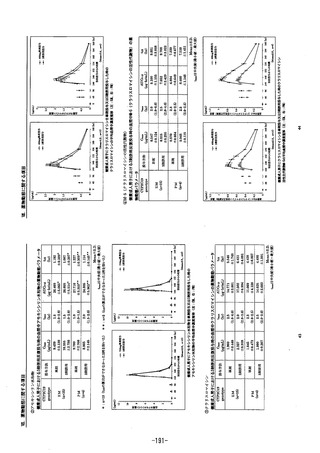
参考資料 (268 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
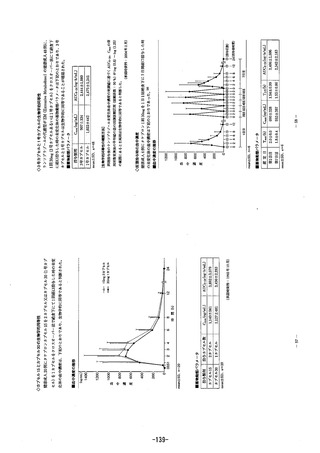
A7なSec7efoy Ac Oral administration of a 20 mg dose of PARIET provides rapid and
ejfective reduction of 9astric acid secretion. The onset of the anti-secretory effect occurs within
one hour with the maxinum effect occurrindg within two to four hours. Inhibition of basal and
food-stimulated acid secretion 23 hours after the first dose of rabeprazole sodium iS 69% and
82% respectively, and the duration of inhibition fasts up to 48 hours. The duraton of
pharmacodynamic action is much longer than the pharmacokinetic half-life (approximately one
hour) would predict. This effect is probably due to he prolonged binding of rabeprazole sodium
to the parietal HK"-ATPase enzyme. The inhibitory effect of rabeprazole sodium on acid
secretion jncreases slightly with repeated once daily dosing, achieving steady state nhibition
after three days. Vhen the drug is discontinued, secretory activity normalises over 2 to 3 dayS.
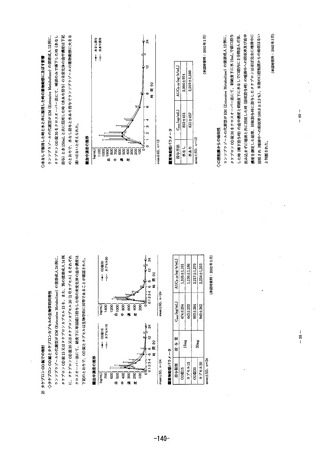
Se Gasが7 放cfS: Im clinical studies, patients were treated once daily wtth 10 or 20 mg
rabeprazole sodium for up to 12 months duration. Serumm gastrjn levels increased during the rst
2 to 8 weeks reflecting the inhibitory effects on acid secretion. Gastrin values returned tO Dr@e-
treatment leveis, usually within 1 to 2 weeks after discontinuation of therapy. In a maintenance
study, which was subsequently extended up to 5 years duration, serum gastrin !evels were only
modestly raised in most patients.
とerocカ7o77a訪た放6 (EC/) Ce7 とがecfS: Increased serum gaStrin secondary to antisecretory
agents stimulates proliferation of gastric ECL cells which, over time, may result in ECL cell
hyperplasia in rats and mice and gastric carcinoids in rats, especlally females (See
Carcinogenicity, Mutagenicity and Impairment of Fertiliy).
In over 400 patients treated with PARIET (10 or 20 mg/day) for up to one year, the incldence of
ECL cell hyperpiasia increased with time and dose, which is consistent with the pharmacologicai
acton of the proton pump Inhibitor. No patient developed the adenomatoid, dysplastic Or
neoplastic changes of ECL cells in the gastric mucosa. No patient developed the carcinoid
tumours observed jn atS.
Pharmnacokinetics
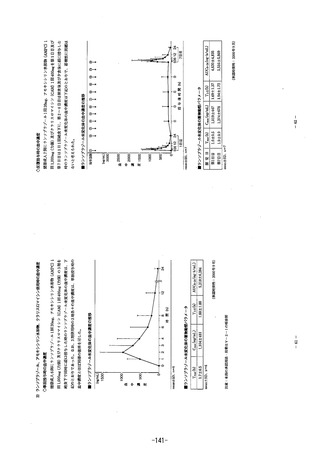
Apso/pがon: PARIET 10 tablets are enteric coated to allow rapeprazole sodium, which jiS acid
jabile, to pass through the stomach intact. Absorption IS rapid, with peak piasma levels of
rabeprazole sodium occurring approximately 3.5 hours after a 20 mg dose. PeaK plasma
concentrations (Ca) of rabeprazole sodium and AUC are Iinear over the dose range of 10 mg
to 40 mg.
Absolute bioavailability of an oral 20 mg dose (compared to intravenous administration) ls apout
52%, largely due to pre-systemic metabolism. Additionally, he bioavailability does not appear to
increase witn repeat administration. In healhy subjects, ine plasma halflife is approximately
one hour (range 0.7 to 1.5 hours) and the total body clearance is estimated to be 283よ 98
mL7mn.
のjS775uがon: Rabeprazole sodium S approximately 979% bound.to human plasIma proteins. Aiter
intravenous administration the voiume of distribution is 0.34 L/Kg.
ルefapo/S77・ Rabeprazole sodium is metabolised through the cytochrome P450 (CYP450)
hepatic drug metabolism system (see Interactions with Other Drugs). In humans, the
thioether (M1) and carboxylic acid (M6) are the main plasma metabolites with the suiphone
(M2), desmethy! thioether (M4) and mercapturic acid conjugate (M5) minor metabolites
observed gt iower levels. Only the desmethyl metabolite (M3) has a smtall amount of ant-
secretory activity, but its presence in plasma is minimal
と廊カ7がo7 an0 Excregon: Following a single 20 mg “C-labelled oral dose of rabeprazole
sodium, no unchanged drud was excreted in the urine. Approximately 907%% of 1he dose was
eiiminated in urine mainly as the two metabolites: a mercapturic acid conjugate (MS5) and a
CarDOxXylic acid (M6), plus two unknown metabolites ajlso found jn 1he specles used jn the
toxicology studies. The rermainder of the dose was recovered in faeces. otal recovery waSs
99.8%. This suggests low biiiary excretion of the metaboltes: with bio-transformation and
urinary excretion of water soluble metabolites as the primary route of eljimination. “
2 PARIET10(1504089)PFi
-304-