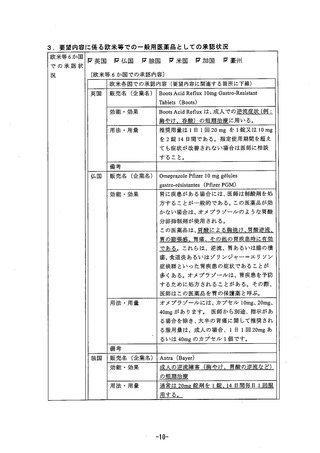
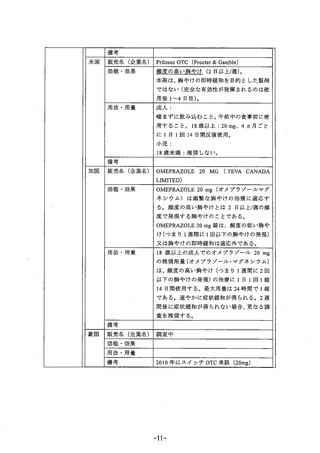
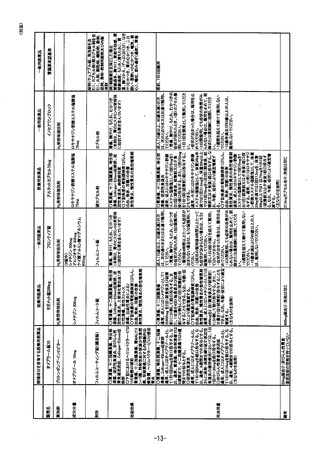
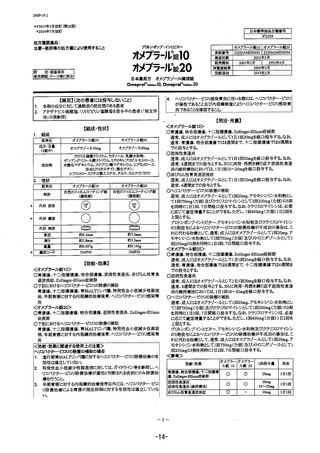
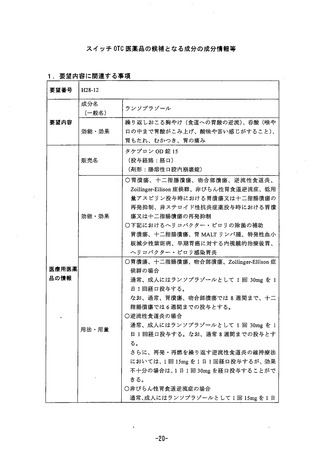
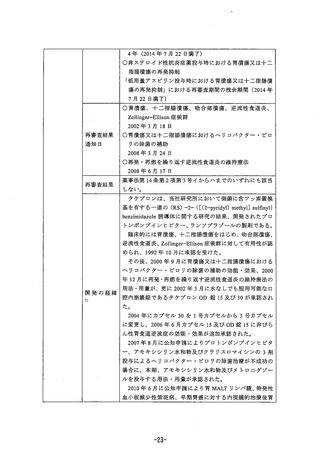
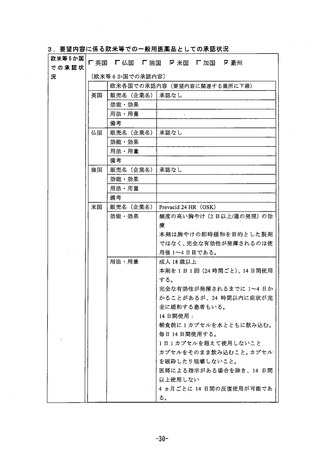
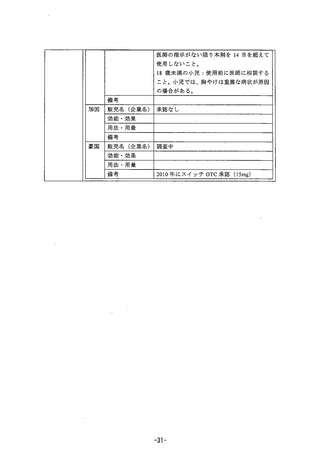
参考資料 (269 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
haemodialysis (creatinine clearance s 5 mLmin/1.73 m2, the pharmacokinetics of rabeprazole
sodium was Very similar to that in healthy vojunteers.
7epafc Dsegse: In a single dose study of 10 patients with chronic mild to moderate
compensated cirrhosjs of the liver who were administered a 20 ma dose of rabeprazole, AUC。>4
was approximately doubied, the elinmination half-life was 2- to 3-fold higher, and totai body
ciearance waS decreased to less tnan haif compared to values in healthy men.
In a multiple dose study of 12 patients with mild to moderate hepatic impairment administered
20 mg rabeprazole once dally for eight days, AUC。.。 and CuAx values increased apDrOximately
302%% compared to values in healthy age- and gender-matched subjects. These increases were
not statistically significant,
No jnforrnation exists on rabeprazole disposition in patients with severe hepatic impairment.
Please refer to the DOSAGE AND ADMINISTRATION section for.information on dosage
adjustments in patents with hepatic impairmert.
Ge7g77CS: Elimination of rabeprazole sodium was decreased in the elderly. Following 7 days of
daily dosing with 20 mg of rabeprazole sodium, the AUC approximately doubled and the Caaz
increased by 60% as compared to youndg healthy volunteers. However, there was no evidence
of rabeprazole sodium accumulation.
CHnical trials
Sア77D7O77dがC Gasな7o-Oesopカageg/ Reガux Prsease (GORP): On-demand treatrment was
assessed in a European Imulticentre, double-blind placebo-controlled randomised withdrawal
study (ロ=418) in endoscopically negative patients.
Following an acute open-label phase, patients were randomised to receive rabeprazole 10 mg
or placebo taken once daily, when required, over a six month period. Efficacy of rabeprazole 10
md on-demand, in patients with complete heartburn relief at baseline was primartly evaluated by
the unwilingness io continue the trial because of inadequate heartburn control. Overall the
proportion of patients discontinuing due to inadequate heartburn control was significantly higher
for placebo (20%) compared to rabeprazole (6%) (p<0.00001).
Patients were instructed to take study drug umtil they had experienced a full 24 hours fiee of ・
heartburn, most patients jn the rabeprazoje group had maximum episode duration of 4 days or
less. In additlon, antacid use was about 2-fold higher in the placebo group than in the
rabeprazoie qrOUD (D=0. 0011). Treatment failure was associated with an increased antacidg
COnSurmDtion.
The efficacy of rabeprazojie 10 mg or 20 mg daily versus piacebo was assessed in a
randomised, double blind, parallel group study (n=199) over a four week interval in subjects with
moderately severe gastro-oesophageal reflux disease and grade 0 or 1 oesophagitis at
endoscopy. 「he primary efficacy variabie was defined as the time in days for subjects to
achieve their first 24 hour intervai without heartburn. Results showed that the average times
were 6.541 +/- 0.923 days for rabeprazole 10 mg, 10 +/- 1.258 days for rabeprazole 20 mg and
16.347 +/ 1.105 days for placebo. There was no significant difference petween the rabeprazole
TOUDS.
INDICATIONS
PARIET 10 js indicated for:
es Symptomatic reiief of heartburn and other symptoms of gastro-oesophageal reflux
disease
CONTRAINDICATIONS
PARIET 10 is contraindicated in patients with known hypersensiivity {to rabeprazoje sodium,
protonl pump Inhibitors, or any ingredient of this product.
3 PARIET10(150409)PFI
-305-