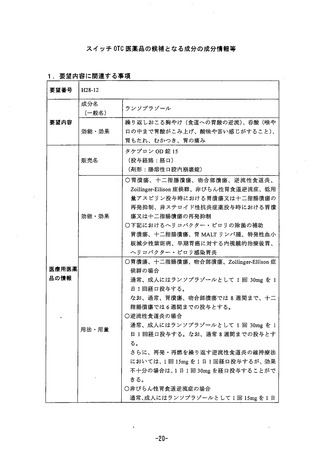
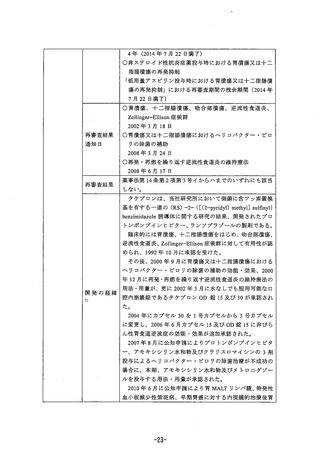
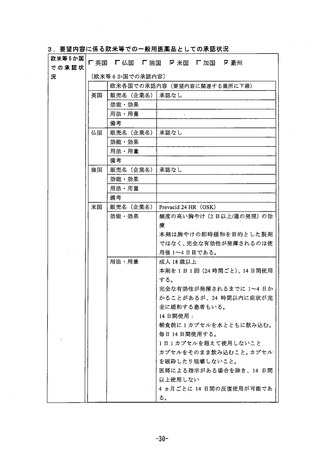
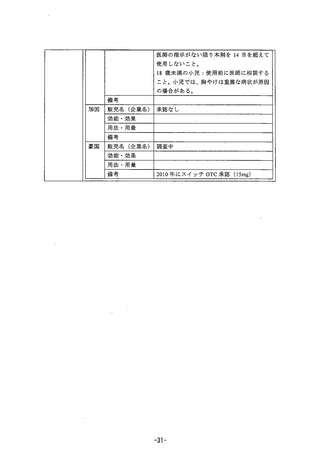
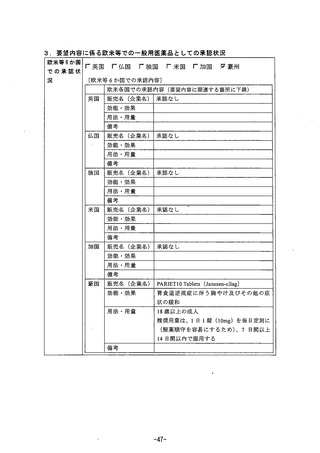
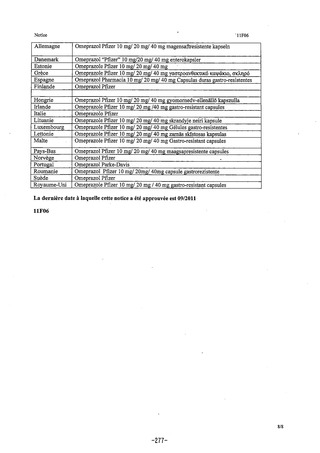
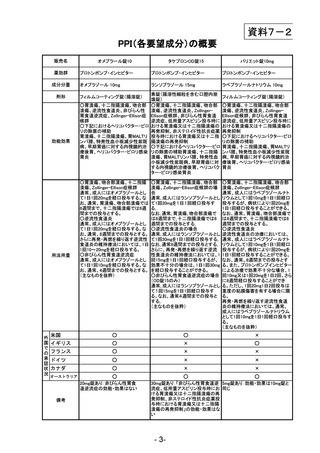
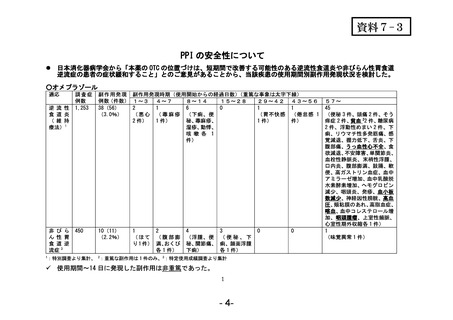
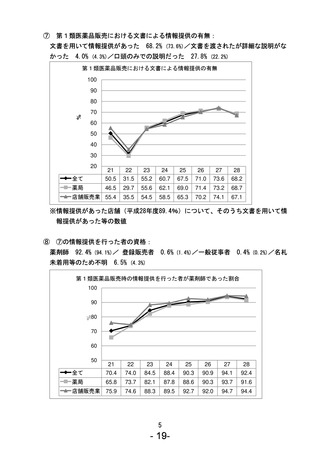
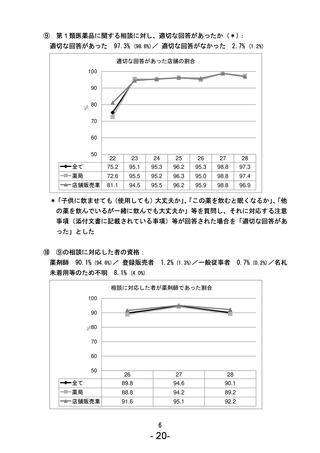
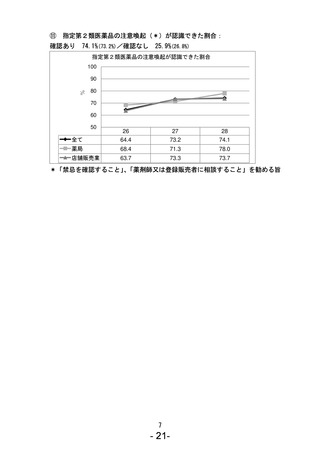
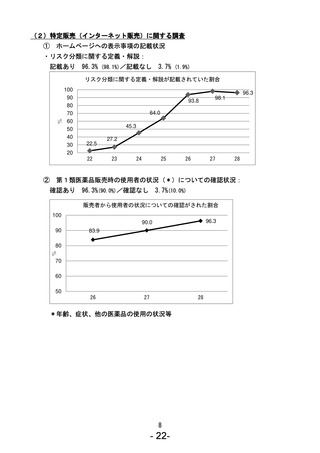
参考資料 (270 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Symptomatic response to therapy with PARIET 10 does not preclude the presence Of gastric
malignancy, therefore the possibility of malignancy should be excluded prior to commencind
treatment with PARIET 10.
Patients on PARIET 10 should be furher reviewed and/or investigated f syrmptoms persist or
recur wiihin 2 weeks of completing the course.
Patients should be referred to fheir doctor for review
es they have unintentional weight loss, anaemia, gastrointestinal bleedind, dysphagia,
persistent vomiting or vomiting with blood, malaena, astric ulcer IS SUspected Or
present or gastrointestinal surgery, as treatment with pantoprazole may alleviate
syhptoms and delay diagnosis. In these cases, malignancy should be excluded.
es {hey have had to take other medication for indigestion or heartburn continuously for
four or more weekS in order to control their symptoms.
e they are being treated for symptomatic GORD and require PARIET 10 for more than
14 days.
ゅe they have any other significani medical condition.
Acufo fe/Sが7がag7 がepカ7が5 “
Acufe の7S大7 epカ77S カaS De67カ Obse/ved 思 paがefS ね9 prOfO7-pC77D 万DがOrS (だ)
のdn9 78Dep79ZO/6 SOd'u77. AcCOB 加好7S7g7 76D太罰S 778y OCC07 97 87y DO77 ug P
訪ergpy 877 /S 9676/dルルコ訪めed fo ag7 Op3訪必 がpe/Se7S店76gC8o7. DSCOカ妨70@
pep/dZO/ら SOu77 CU 7f9/Sg7g/ ep用/77S Ve/ODS.
*CyanOCOわgg) (Vコ7777 硬-72) DeがCc7encY
りa放 がeaが7e/77 W訪 9C/O-SUDD7@SS有9 7767C76S OV67 8 /O79 De7Od 0Oげ 刀76 (9.7. /O7967 妨7 ば
Yeg7S) 9y /ら9 7O 8DSO/のが77 0げ7 cyg7OCOカg褒7加 (の記軌太 B-72) caused py カYDO- Or
CO旋YO7g.
Use in Patients with Hepatic Impairment.
No dosage adjustment is necessary for patients with hepatic impairment. While no evidence of
significant drug related safety problems was observed in patients with hepatic impalrment, it [IS
advised to exerclse caution when ireaiment with PARIET 10 js fst initiated jn patiehts with
severe hepatic dysfunction (see DOSAGE & ADMINISTRATION).
Patients should be referred to thejr doctor for review if they have severe hepatic Impairment (e9.
CfrhOSIS).
HypomagneSseimia
Hypomagnesemia, Symptomatic and asymptomatic, has been reported rarely In patients treaied
with PPls. Serious adverse events include tetany, arrhythmias, and seizures. In Inost patients,
treatment of hypomagnesermia reduired maqnesium replacerment and discontinuation of the
PPI.
For patients expectedl to be on prolonged treatment or who take PPls with medications such as
diaoxin or drugs that may cause hypomadgneseimia (e.9. diuretics), health care professionals
may consider monitoring magnesium levels prior 1o initiation of PPI treatment and then
periodjically while freatment coniinues (see ADVERSE REACTIONS).
FractuireSs
Observational studies suggest that proton pump inhibitor (PP!) therapy may be associated with
an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture
was increased in patients who received high-dose, and Iong-term PPI iherapy (a year or ionger).
4 PARIET10(150409)PPI
-306-