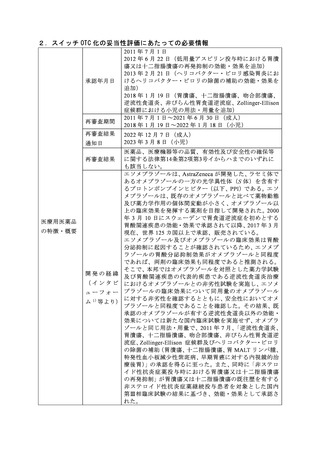
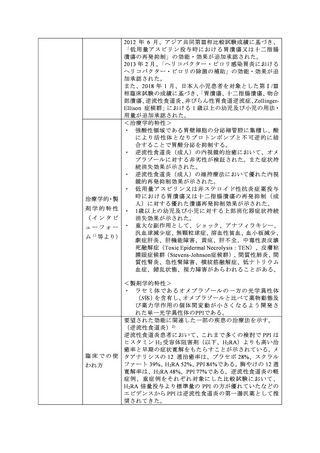
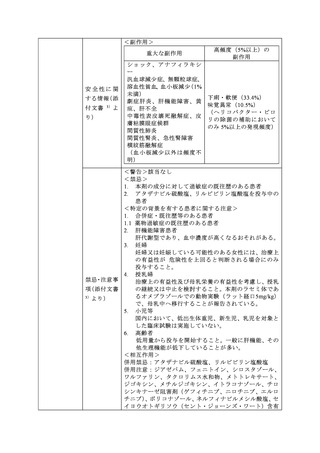
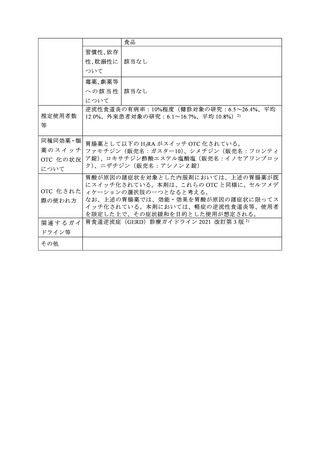
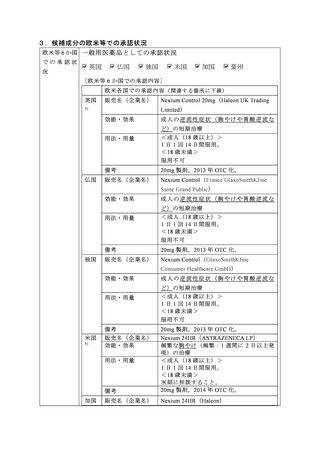
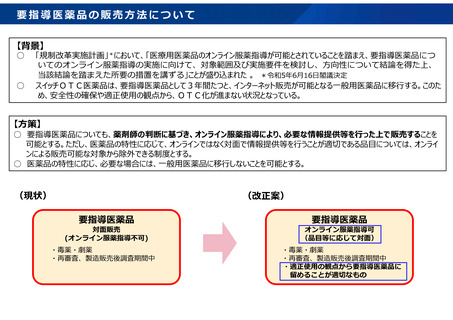
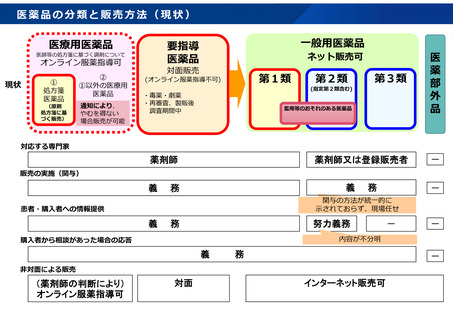
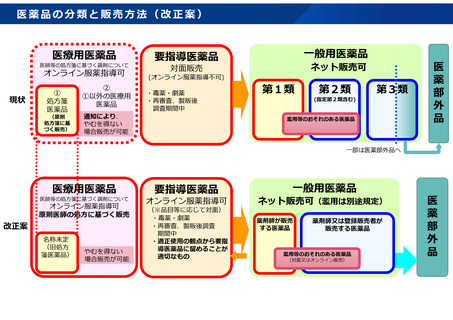
参考資料 (273 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
by 24% and 50% respectively during concormitant administration. ThiS is considered to be a
useful interaction durind 刀 pyWo7 eradication.
ADVERSE REACTIONS
Rabeprazole was generally well tolerated during clinical trials. The observed side effects have
geherally been mild or moderate and transient in nature. In the majority of cases, the Incidence
of the adverse events in the rabeprazole treatment group was equal to or less than that
observed in the placebo control treatment qrouD.
Only headaches, diarrhoea, abdominal pain, asthenia, fiatulence, rash and dry mouth have
been associated with the use of rabeprazole.
The adverse .events, which may or may'rnot be causally related to rabeprazole, reported in
cojinical ffjals are listed below in descending order of frequency.
Common (> 196 and < 102%)
eyんoO/S SyS7fe77- headache, dizzineSS.
GSか077@S妨7た diarrhoea, nausea, abdominal pain, flatuience, vomiiting, constipation.
ReSD73fOn/ fhintis, pharyngitis, cough.
M7uscugosKe/らfg non-spbecific pain, back pain, Imyaldia.
Ioイル raSh.
〇のer asthenia, fulike syndrome, infection, insomnia, chest pain.
Uncommon (= 0.19% and < 1%)
GSかの加放S妨7 dyspepsia, eructation, dry mouth.
Resp7gfo77: sinusitis, bronchitis.
Msc/osKe/らね/ 。 arthralgia, leg cramDps.
び7778が: urinary tract infection.
Oのer fever, nervousness, somnolence, chills, peripheral oedema.
Rare (と 0.017% and < 0.42)
G8S#かり記妃S妨78 anorexla, gastritis, weight gain, stomatitis.
らルた77 Druritis, Sweatind.
SO@o/g/ S@7SeS: VISIOn or fgste disturbances.
geの8fO/O97C: leucOCytoSISs.
O妨@ deDreSsSsiOonl.
Post-Marketing Experience
Erythema and rarely bullous reactions, urticarial skin eruptions and acute systemic allergic
reactions, for example facial sweiing, hypotension and dyspnoea have been reported in
patients treated with rabeprazole. These usually resolved after discontinuation of theraPy.
Erytnema muliforme, Interstitial nephrits, gynaecomastia, myalgia and potential alergic
reactions including anaphylactic reactions have been reported rarely. Blood dyscrasia including
thrombocytopenia, neutropenla, jeGukopenia, pancytopenia, agranulocytosis and bicytopenia
have been reported rarely. Hypomagnesermia has also been jeported rarely.
There have aiSo been reports of increased hepatc enzymes and serious nepatic dysfunction
such as hepatitis and jaundice. Rare reports of hepatic'encephalopathy have been received in
paiients with underlyihd clrrhoSsiS.
There have been very rare reDorts of toxic epidermal necrolysis (TEN) and Stevens-Johnson
Syndrome.
There have been posi-rmarketing reports of bone fractures (see PRECAUTIONS).
7 PARIET10(150409)PPI
~-308-