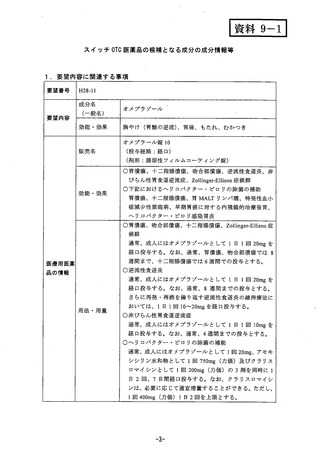
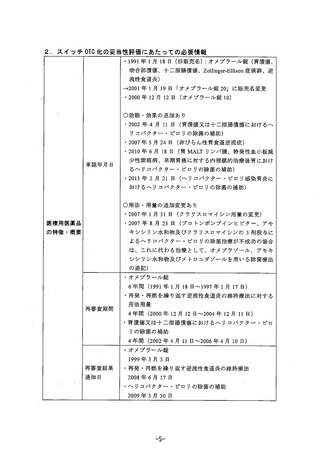
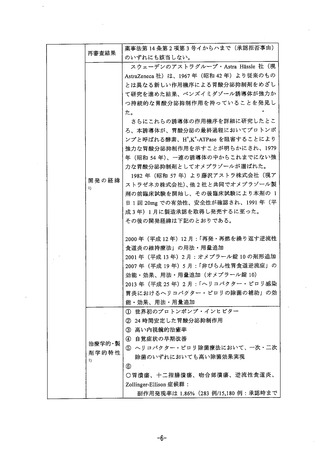
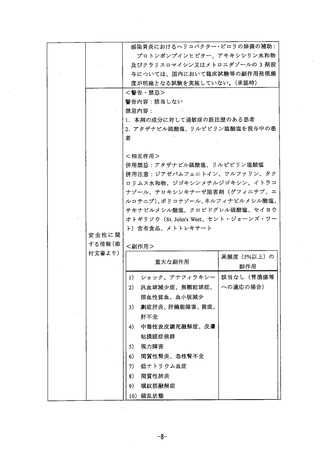
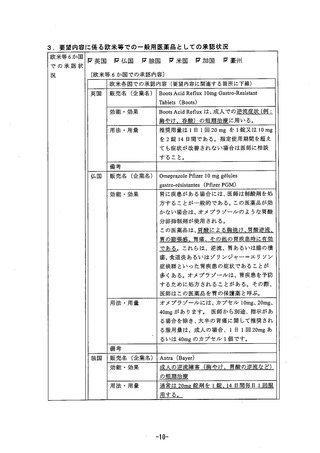
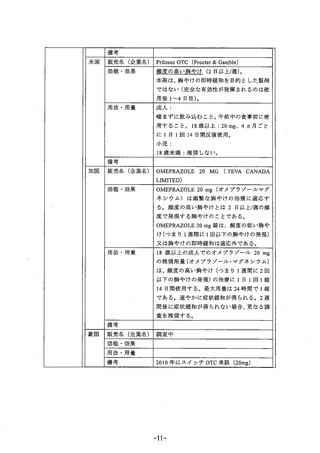
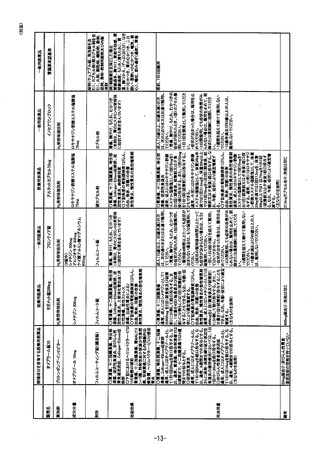
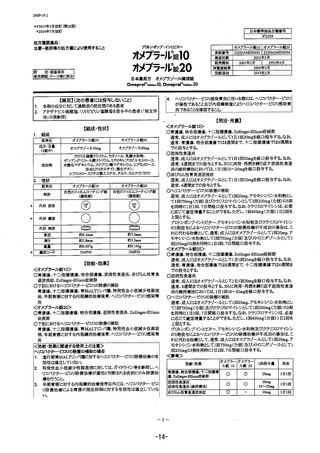
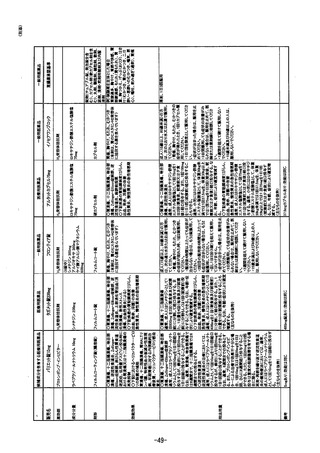
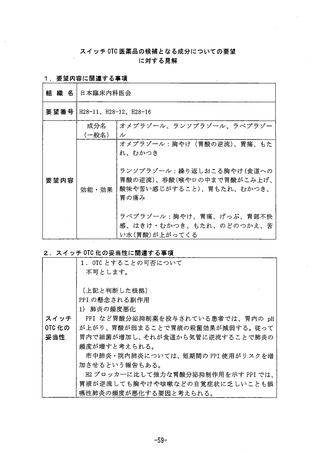
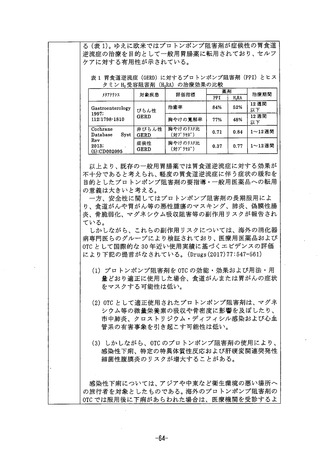
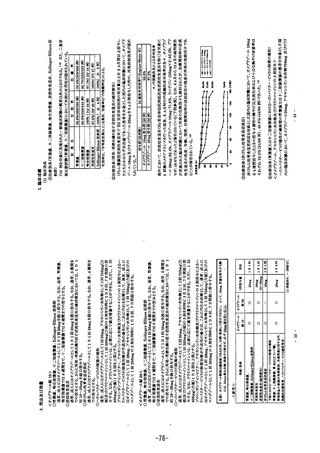
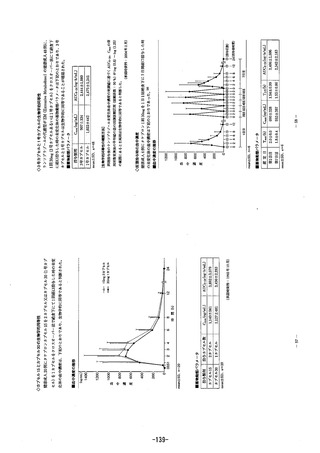
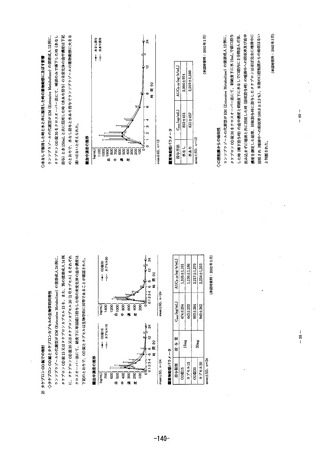
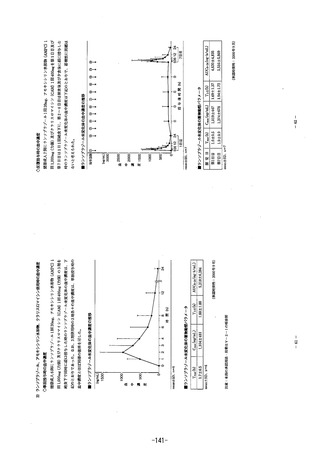
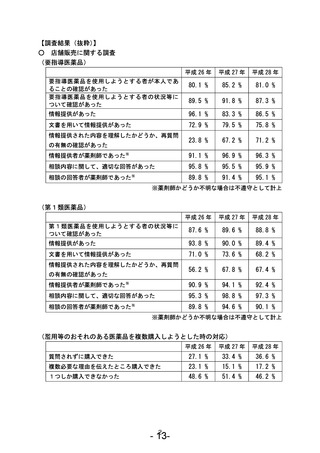
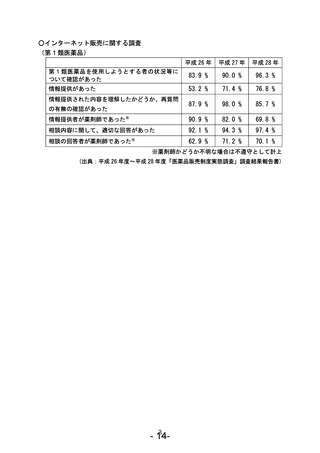
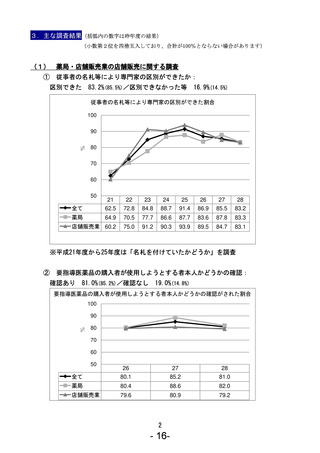
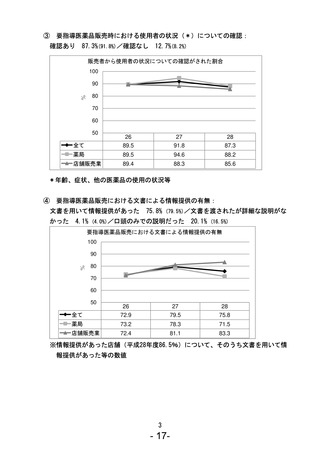
参考資料 (271 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
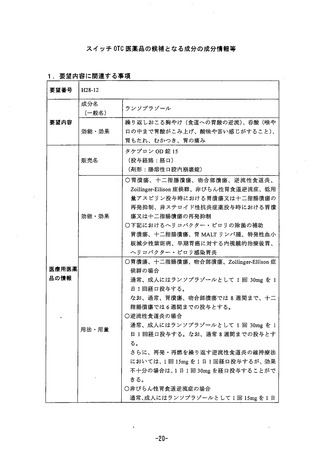
Literature suggests that concormitant use of PPls with methotrexate (primarily at high dosei: see
methoirexate prescribing information) may elevate and prolong serum levels of metnotrexate
and/or its metabolite, possibly jeading to methotrexate toxicities. In such high-doses
methotrexate administration, a temporary withdrawal of the PPI may be considered in some
patients.
Clostridiurm difficile
Treatment with proton pump inhibitors may possibly increase the risk of gastrointestinal
infections such as Cosが7 万B.
Carcinogenicity, Mutagenicity and Impairment of Fertility
ofe: In the following section, he relative exposure levels in animals have been calculated USInQd
human dose of 20mg/day.
In an 687104 week carcinogenicity study in CD-1 mice, rabeprazole at oral doses up to 100
Img/kg/day did not produce any increased tumour occurrence. The highest tested dose
produced a systemlc.exposure to rabeprazole (AUC) of 1.40 ng.hymL which is 1.6 times the
human exposure 4t 20 mg/day.
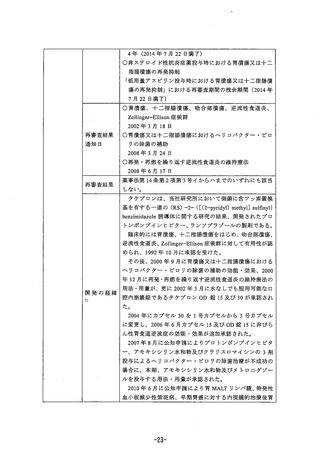
In a 104-week carcinogenicity study in SD rats, males were treated with oral doses of 5, 15, 30
and 60mg/kg/day and females with 5, 15, 30, 60 and 120 mg/kg/day. Rabeprazole produced
gastric enterochromaffin-like (ECL) cell hyperplasia im male and female rats and ECL cel
carcinoid tumours in female rats at al doses. The lowest dose (5 mg/kg/day) produced a
systemic exposure to rabeprazole (AUC) of about 0.1 ug.hrmsL which is about 0.1 times the
human exposure at 20 mgday. In maie rats, no treatment-related tumours were observed at
doses up to 60 mag/kg/day producing a rabeprazole plasma SXpOSure (AUC) of about 0.2
Hg.hrrrL (0.2 times the human exposure at 20 mg/day).
Rabeprazole was positive in assays for gene mutations (the AMES test, forward gene mutation
tests in Chinese namster ovary cells (CHO/HGPRT) and mouse Iymphoma cells (L5178Y/TK+/-
). ItS demethylated-metapolite was also positive in the AMES test. Rabeprazole was negative
in assays for chromosomal damage (tne 訪 zo Chinese hamster lung cell chromosome
aperration test, the 妨 vo mouse micronucleus test), and 罰 Vo and ex Wvo rat hepatocyte
unscheduled DNA synthesis (UDS) tests.
Rabeprazole at infravenous doses up to 30 mqg/kg/day (plasma AUC of 8.8 ig.hrmL, about 10
times the human exposure at 20 mg/day) was found to have no effect on fertiity and
reDproductive performance of male and female rats.
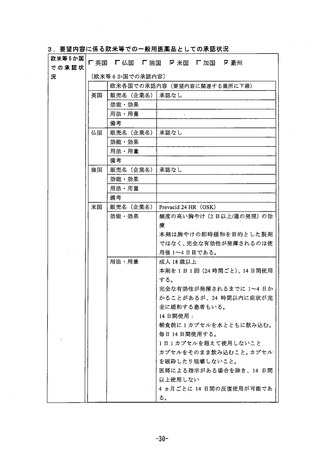
Use in Pregnancy
Cateqgory B1.
Teratology studies have been performed in rats at intravenous doses up to 50 mokg/day
(plasma AUC of 11.8 Jug.hrmL, about 13 or 6.5 times the human exposure at 20 mg/day and
40mg/day respectively, and rabbits at intravenous doses up to 30 mg/kq/day (plasma AUC of
7.3 ng.hrmL, about 8 or 4 fimes the human exposure at 20 mg/day and 40mg/day respectivejy)
and have revealed no evidence of impaired fertility or harm to the foetus due to rabeprazole.
There are no adequate and welcontrolled studies in pregnant women and post-marketing
experience IS very limited. Rabeprazole sodium should be used in pregnancy only ff the
potential benei justiies the potential risk to the foetus.
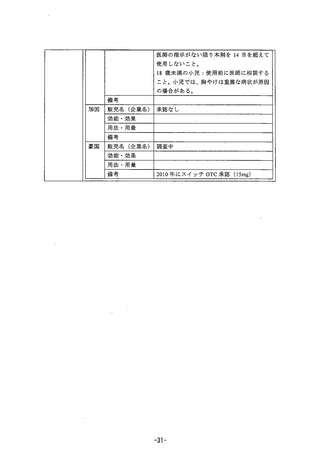
Use in Lactation
Following intravenous administration of “C-labelied rabeprazole to lactating rats, radioactivity in
milk reached levels that were about 2- to 7-fold higher than jevels in the blood. Administration of
rabeprazole to rats in gestation and during lactation at doses of 400 mg/kg/day (about 195-or
85-times a 20mg or 40mg human dose based on mg/m?) reSulted in decreases jn body weight
aain of the DUDS.
5 PARIET10(150409)PP!
-307-