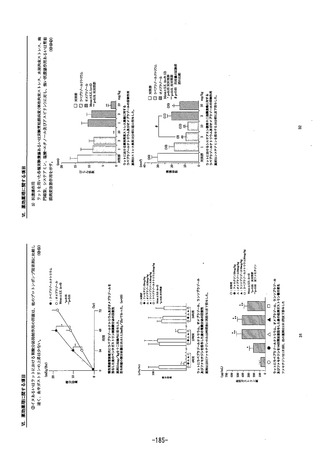
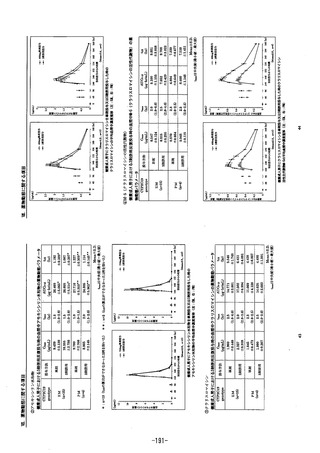
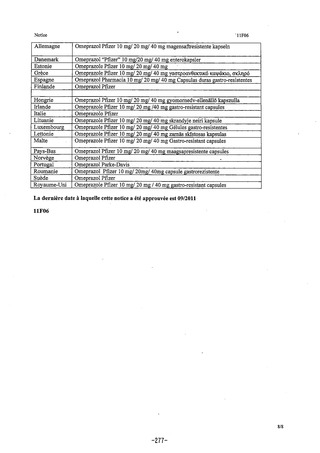
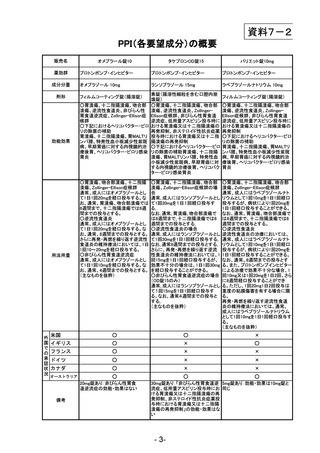
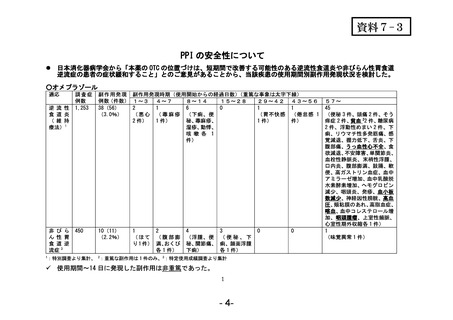
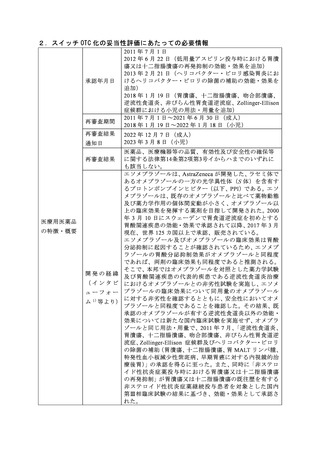
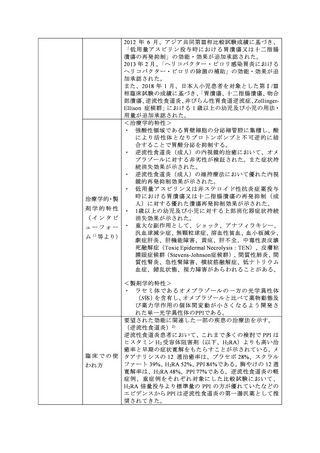
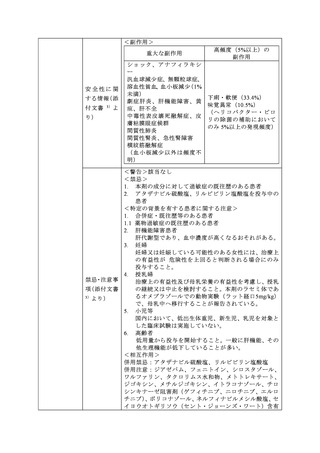
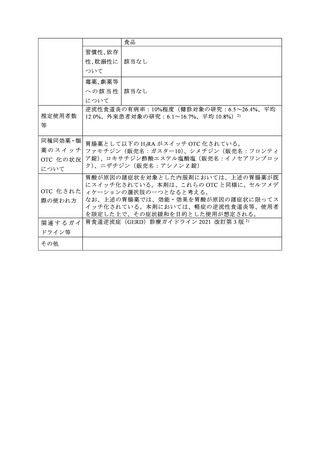
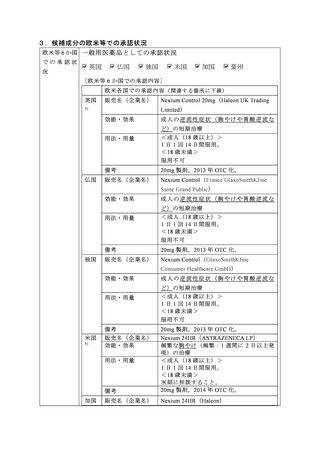
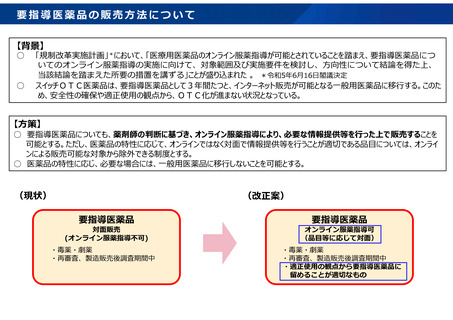
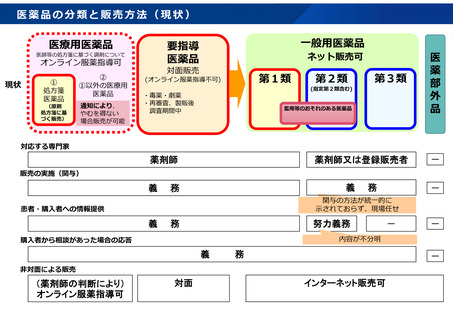
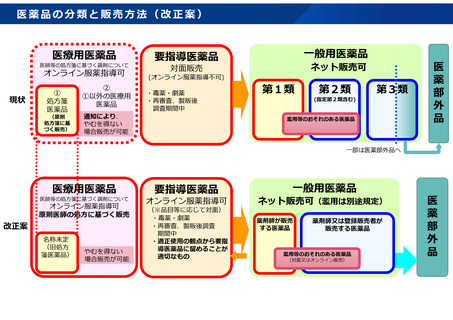
参考資料 (274 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198111_00025.html |
| 出典情報 | 医療用から要指導・一般用への転用に関する評価検討会議(第27回 3/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Adiults 18 years of age and over:
The recommended dose is one tablet per day to be taken at ihe same ime each day (to
facilitate treatment compiliance) for at least 7 days and up to 14 days. If symptom control has
not been achieved after two weeks of continuous treatment with PARIET 10, patients should be
referred to their doctor. PARIET 10 tablets should not be chewed or crushed, but should be
Swallowed whole. PARIET 10 tablets were taken with or without food jn the pivotal clinical trials.
Use in Children under 18 years of age
PARIET 10 js not recommended for use in children as there is no experiertce of ItS use In fhiS
droUP. “
Use in Elderly Patients
No dosage adjustment is necessary in elderly patients.
Use in Patients wtth Hepatic or Renal Impairment
No dosage adjustment is necessary for patients wtn renal impairment. Patients with mild to
moderate hepatic impairment experience higher exposure to rabeprazole sodium at a given
dose than do heaithy patients. Caution should be exercised in patients with severe hepatic
impairment (see PRECAUTIONS).
OVERDOSAGE
Experience to date wifh deliberate or accidental overdose iS liimited. he maximum established
exposure has not exceeded 60 mg twice daily, or 160 mg once dally. Effects are generally
minimal, representative of the known adverse event profile, and reversible without furher
medical interventlon. No specific antidote is known. Rabeprazole sodium is extensively Drotein
bound and js therefore not readily dialysable. As in any case of overdose, treatmient should be
symptomatic and general suppoirtive measures should be used.
. PRESENTATION AND STORAGE CONDITIONS
PARIET 10 tablets are pink, biconvex tablets, marked with “"E 241” jn biack ink on one side.
Supplied jn blister packs of 7 and 14 fablets.
Storage Conditions
PARIET 10 tablets should be stored below 25"C. Do not refrigerate or freeze.
Tabjets are presented in an aluminium/ajuminium blister. (
NAME AND ADDRESS OF THE SPONSOR
Janssen-Cilag Pty Ltd.
1-5 Khartoum Rd
Macquarie Park NSW 2113
Australia
POISON SCHEDULE OF THE MEDiCINE
Schedule 3- Pharnmacist Oniy Medicine.
DATE OF FIRST INCLUSION IN THE ARTG
01 April 2010
8 PARIET10(150409)PPI
-310-