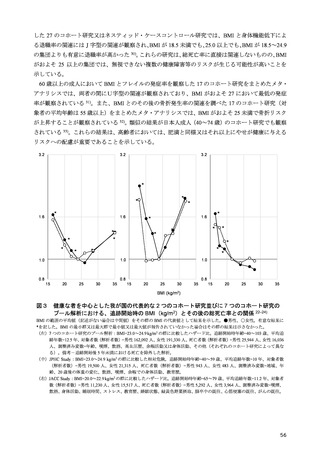
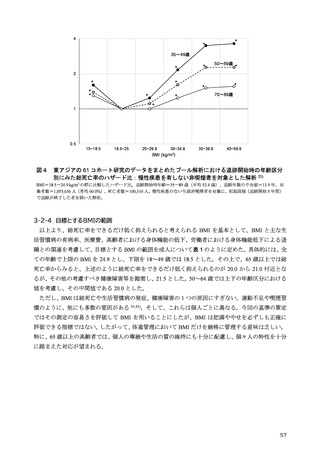
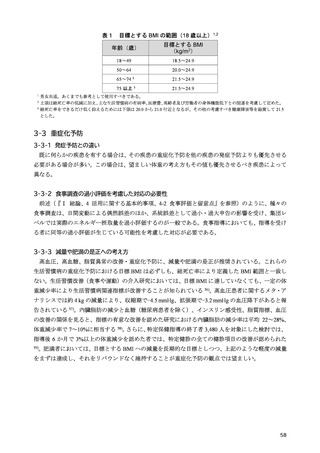
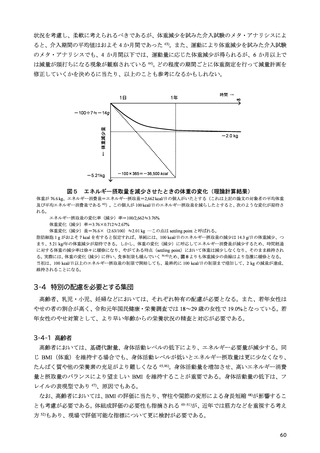
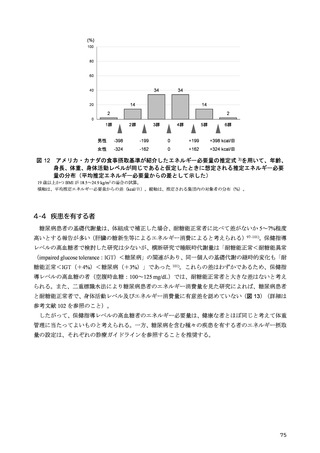
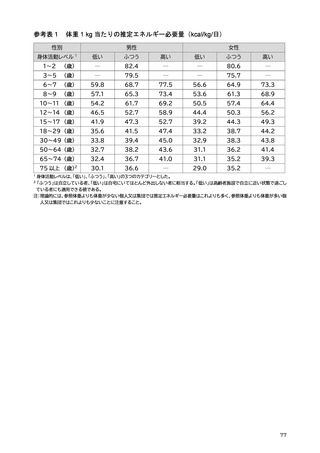
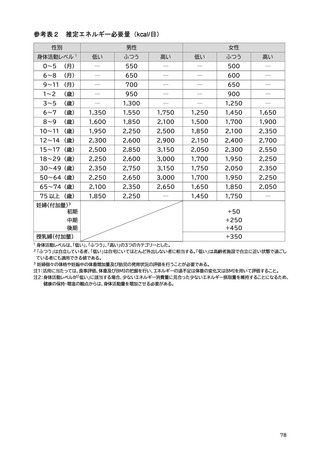
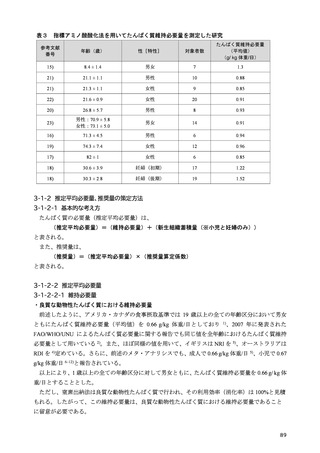
「日本人の食事摂取基準(2025年版)」策定検討会報告書 (187 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_44138.html |
| 出典情報 | 「日本人の食事摂取基準(2025年版)」策定検討会報告書(10/11)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
75) Fomon SJ, Younoszai MK, Thomas LN. Influence of vitamin D on linear growth of normal full-term infants.
J Nutr. 1966;88(3):345-350.
76) EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel), Turck D, Bresson JL, et al.
Update of the tolerable upper intake level for vitamin D for infants. EFSA J. 2018;16(8):e05365.
77) Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind,
randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341-2357.
78) Tamaki J, Iki M, Sato Y, et al. Total 25-hydroxyvitamin D levels predict fracture risk: results from the 15year follow-up of the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos
Int. 2017;28(6):1903-1913.
79) Ju SY, Lee JY, Kim DH. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: a systematic
review and dose-response meta-analysis. BMC Geriatr. 2018;18(1):206.
80) Wang X, Hu J, Wu D. Risk factors for frailty in older adults. Medicine (Baltimore). 2022;101(34):e30169.
81) Shimizu Y, Kim H, Yoshida H, et al. Serum 25-hydroxyvitamin D level and risk of falls in Japanese
community-dwelling elderly women: a 1-year follow-up study. Osteoporos Int. 2015;26(8):2185-2192.
82) Kong SH, Jang HN, Kim JH, et al. Effect of vitamin D supplementation on risk of fractures and falls
according to dosage and interval: A meta-analysis. Endocrinol Metab (Seoul). 2022;37(2):344-358.
83) Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
84) Kelleher J, Losowsky MS. The absorption of alpha-tocopherol in man. Br J Nutr. 1970;24(4):1033-1047.
85) Blomstrand R, Forsgren L. Labelled tocopherols in man. Intestinal absorption and thoracic-duct lymph
transport of dl-alpha-tocopheryl-3,4-14C2 acetate dl-alpha-tocopheramine-3,4-14C2 dl-alpha-tocopherol(5-methyl-3H) and N-(methyl-3H)-dl-gamma-tocopheramine. Int Z Vitaminforsch. 1968;38(3):328-344.
86) Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Annu Rev Nutr. 1999;19(1):343-355.
87) Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel
mechanism of regulation of vitamin E status. J Biol Chem. 2002;277(28):25290-25296.
88) Horwitt MK, Century B, Zeman AA. Erythrocyte survival time and reticulocyte levels after tocopherol
depletion in man. Am J Clin Nutr. 1963;12:99-106.
89) Andersen LF, Solvoll K, Johansson LR, et al. Evaluation of a food frequency questionnaire with weighed
records, fatty acids, and alpha-tocopherol in adipose tissue and serum. Am J Epidemiol. 1999;150(1):75-87.
90) Horwitt MK. Vitamin E and lipid metabolism in man. Am J Clin Nutr. 1960;8:451-461.
91) Thurnham DI, Davies JA, Crump BJ, et al. The use of different lipids to express serum tocopherol: lipid
ratios for the measurement of vitamin E status. Ann Clin Biochem. 1986;23 ( Pt 5):514-520.
92) Schultz M, Leist M, Petrzika M, et al. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J
Clin Nutr. 1995;62(6 Suppl):1527S-1534S.
93) Lebold KM, Ang A, Traber MG, et al. Urinary α-carboxyethyl hydroxychroman can be used as a predictor
of α-tocopherol adequacy, as demonstrated in the Energetics Study. Am J Clin Nutr. 2012;96(4):801-809.
94) Imai E, Tsuji T, Sano M, et al. Association between 24 hour urinary α-tocopherol catabolite, 2,5,7,8tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman (α-CEHC) and α-tocopherol intake in intervention and
cross-sectional studies. Asia Pac J Clin Nutr. 2011;20(4):507-513.
177