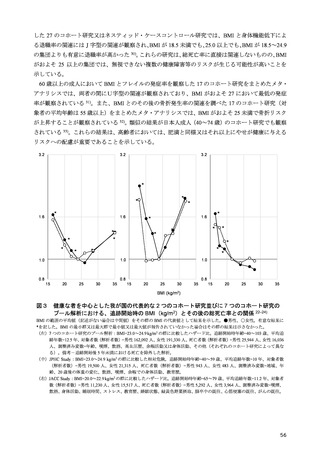
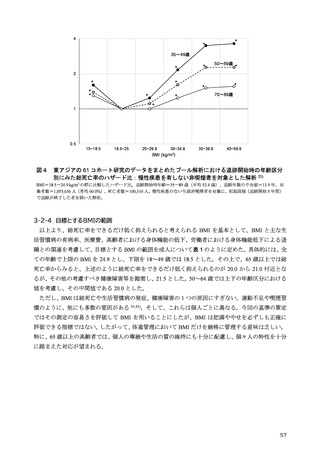
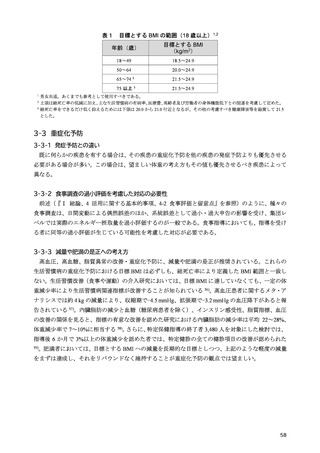
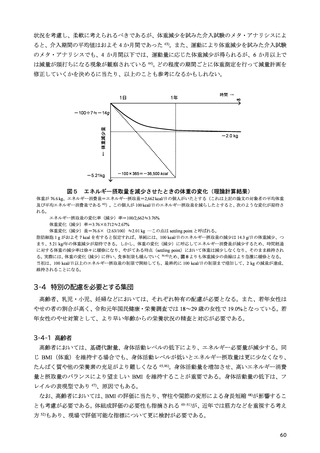
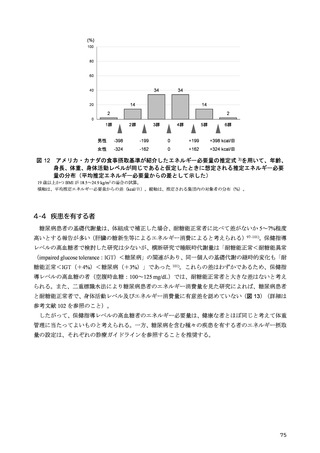
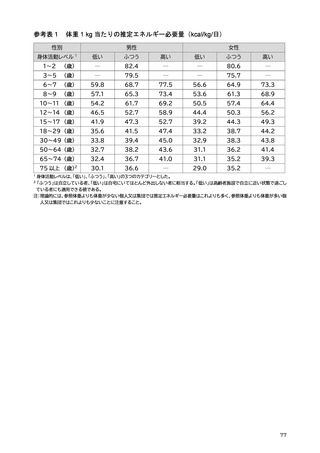
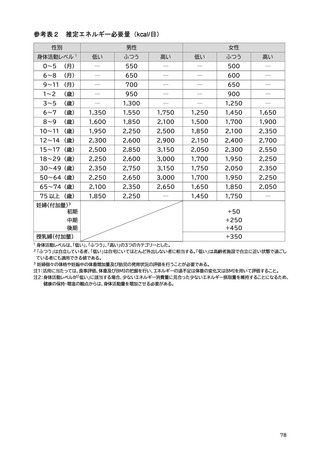
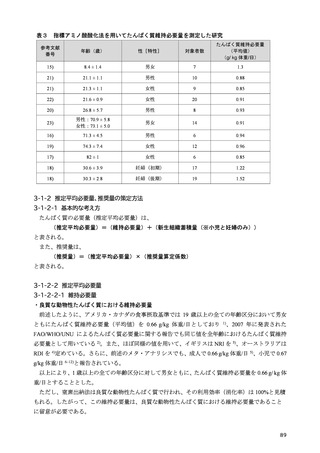
「日本人の食事摂取基準(2025年版)」策定検討会報告書 (353 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_44138.html |
| 出典情報 | 「日本人の食事摂取基準(2025年版)」策定検討会報告書(10/11)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
224) Outridge PM, Scheuhammer AM. Bioaccumulation and toxicology of chromium: implications for wildlife.
Rev Environ Contam Toxicol. 1993;130:31-77.
225) Masharani U, Gjerde C, McCoy S, et al. Chromium supplementation in non-obese non-diabetic subjects is
associated with a decline in insulin sensitivity. BMC Endocr Disord. 2012;12(1):31.
226) Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism
and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30(8):2154-2163.
227) Iqbal N, Cardillo S, Volger S, et al. Chromium picolinate does not improve key features of metabolic
syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7(2):143-150.
228) Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety
of chromium supplementation in diabetes. J Clin Pharm Ther. 2014;39(3):292-306.
229) Zhao F, Pan D, Wang N, et al. Effect of chromium supplementation on blood glucose and lipid levels in
patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Biol Trace Elem
Res. 2022;200(2):516-525.
230) Rajagopalan KV. Molybdenum: an essential trace element in human nutrition. Annu Rev
Nutr. 1988;8(1):401-427.
231) Johnson JL, Waud WR, Rajagopalan KV, et al. Inborn errors of molybdenum metabolism: combined
deficiencies of sulfite oxidase and xanthine dehydrogenase in a patient lacking the molybdenum cofactor.
Proc Natl Acad Sci U S A. 1980;77(6):3715-3719.
232) Abumrad NN, Schneider AJ, Steel D, et al. Amino acid intolerance during prolonged total parenteral
nutrition reversed by molybdate therapy. Am J Clin Nutr. 1981;34(11):2551-2559.
233) Turnlund JR, Keyes WR, Peiffer GL. Molybdenum absorption, excretion, and retention studied with stable
isotopes in young men at five intakes of dietary molybdenum. Am J Clin Nutr. 1995;62(4):790-796.
234) Turnlund JR, Weaver CM, Kim SK, et al. Molybdenum absorption and utilization in humans from soy and
kale intrinsically labeled with stable isotopes of molybdenum. Am J Clin Nutr. 1999;69(6):1217-1223.
235) Yoshida M, Hattori H, Ota S, et al. Molybdenum balance in healthy young Japanese women. J Trace Elem
Med Biol. 2006;20(4):245-252.
236) Turnlund JR, Keyes WR, Peiffer GL, et al. Molybdenum absorption, excretion, and retention studied with
stable isotopes in young men during depletion and repletion. Am J Clin Nutr. 1995;61(5):1102-1109.
237) Institute of Medicine. Molybdenum. In: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic,
Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc.
National Academies Press, Washington D.C.; 2001:420-441.
238) Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake
Level of Molybdenum. European Commission, Brussels; 2000.
239) Fungwe TV, Buddingh F, Demick DS, et al. The role of dietary molybdenum on estrous activity, fertility,
reproduction and molybdenum and copper enzyme activities of female rats. Nutr Res. 1990;10(5):515-524.
240) World Health Organization, International Atomic Energy Agency, Food and Agriculture Organization of
the United Nations. Molybdenum. In: Trace Elements in Human Nutrition and Health. World Health
Organization, Geneva; 1996:144-154.
241) 吉田宗弘, 伊藤智恵, 服部浩之, 他. 日本における母乳および調整粉乳中のモリブデン濃度と乳
343